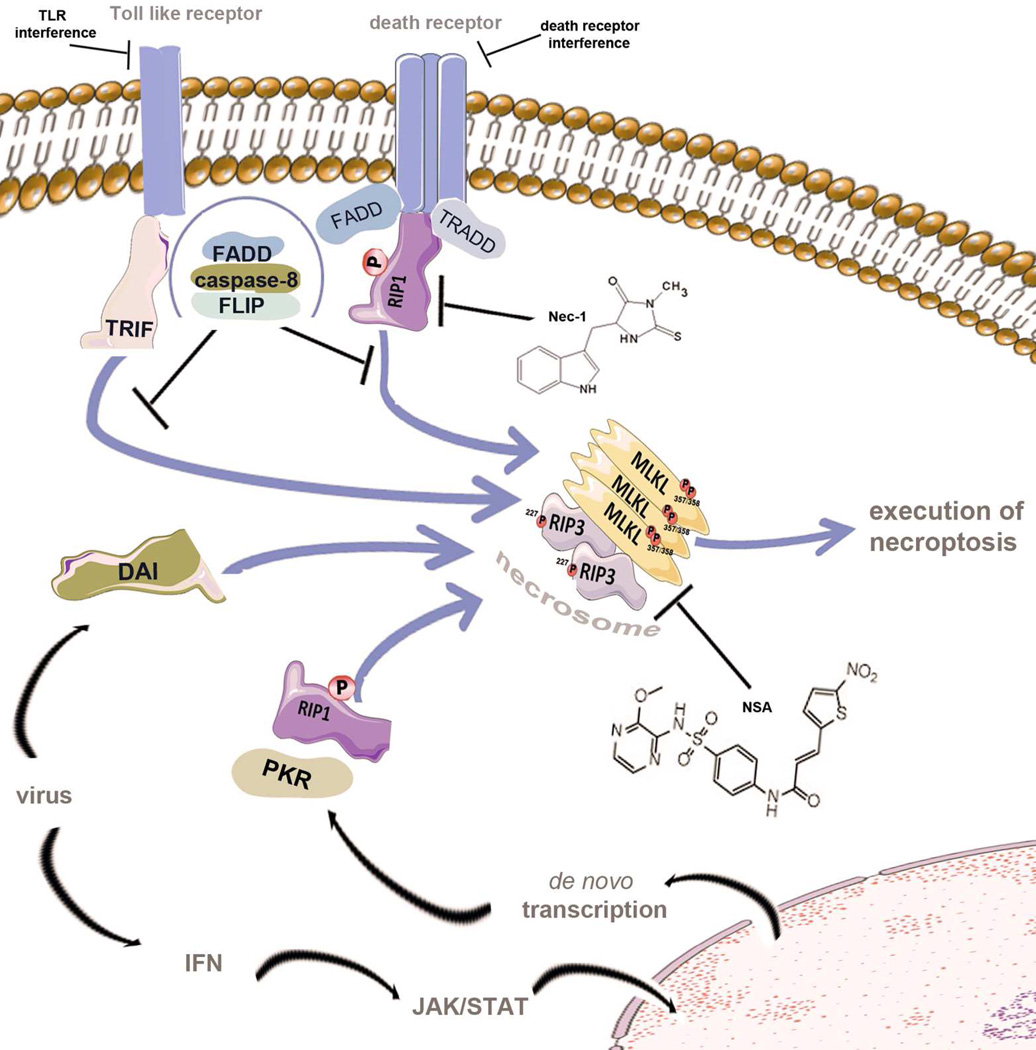
Figure 2. Activators of the necrosome as therapeutic targets.
Various stimuli lead to the activation of the supramolecular necroptosis-inducing complex, referred to as the necrosome. Initially, studies of necroptosis employed models of death receptor stimulation in the presence of caspase-inhibition (not shown). The intracellular adapter molecules FADD and TRADD recruit RIPK1, which subsequently undergoes an incompletely understood series of ubiquitylation, deubiquitinylation, and phosphorylation events before exposing its rip homotypic interaction motif (RHIM-domain) to recruit RIPK3. RIPK1, RIPK3 and MLKL are phosphorylated during the assembly of the necrosome. Within the human genome, RIPK1, RIPK3 and two other proteins exhibit RHIM-domains. One of these is TRIF, an intracellular signal transducer that is capable of activating the necrosome downstream of Toll-like receptors which are triggered by microbial molecules. The fourth RHIM-domain protein, DAI, integrates signals from viral RNA sensors into the necrosome. Finally, viral infection is accompanied by production of interferon, which triggers the JAK/STAT-dependent de novo synthesis of protein kinase R (PKR) which phosphorylates FADD to directly interact with RIPK1 and induce necrosome formation.
Death receptor-mediated necroptosis involves deubiquitinylation of RIPK1, the kinase domain of which is targeted by necrostatin-1 (Nec-1). Second generation RIP1-kinase inhibitors, such as Nec-1s, a stable version of Nec-1 which is more potent at lower concentrations, might reduce observed side effects. Necrosulfonamide (NSA) inhibits MLKL and prevents the activity of the necrosome in human cells. In addition, RIPK3-inhibitors, death receptor antagonists or plasma membrane channel blockers might be attractive therapeutical targets.