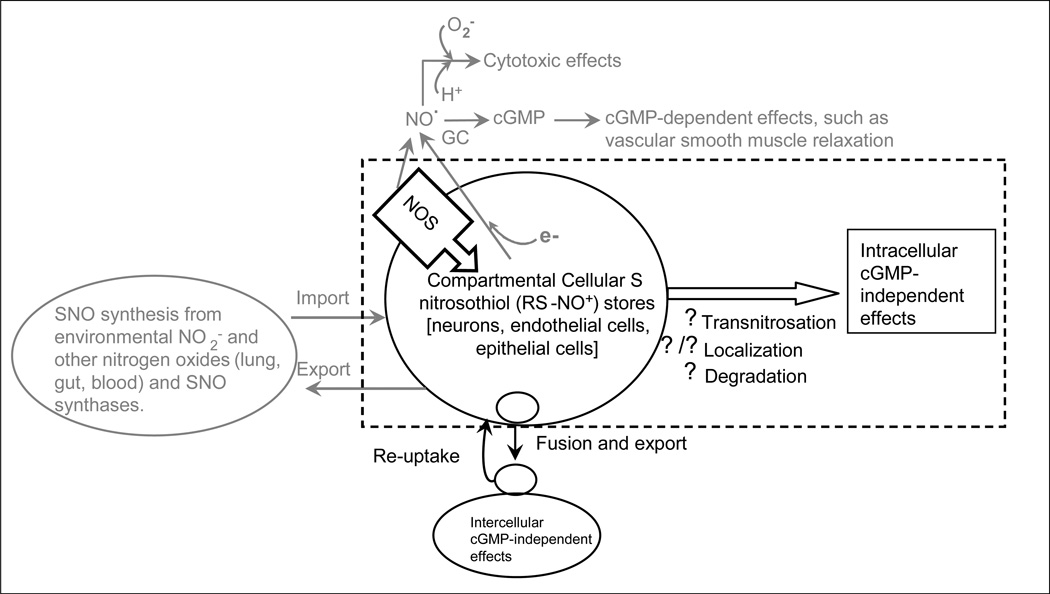
Figure 1. Scientific overview: An emerging paradigm of nitrogen oxide signaling.
Nitric oxide synthase (NOS) forms S-nitrosothiols (RS˙-˙NO, or, more commonly, RS−−NO+). Note there is also regulated cellular import of extracellular S-nitrosothiols. NOS-derived NO and NO reduced from S-nitrosothiols and NO2− can exert classical cytotoxic and cyclic GMP (cGMP)-dependent effects, the latter through activation of guanylyl cyclase (GC). Intracellular S-nitrosothiols can include protein and low-mass species, and are generally in sequestered locations in the cell, such as membranes and vesicles. These S-nitrosothiols can transfer NO+ equivalents to target proteins through transnitrosylation to cause cGMP-independent effects; this signaling can be regulated by movement of the S-nitrosothiols in the cell to target locations, and by degradation. Dr. Lewis’ recent data suggest that S-nitrosothiols can also be secreted into the extracellular space to signal intercellular, cGMP-independent effects—particularly in the autonomic nervous system—through extrusion from S-nitrosothiol-containing vesicles.