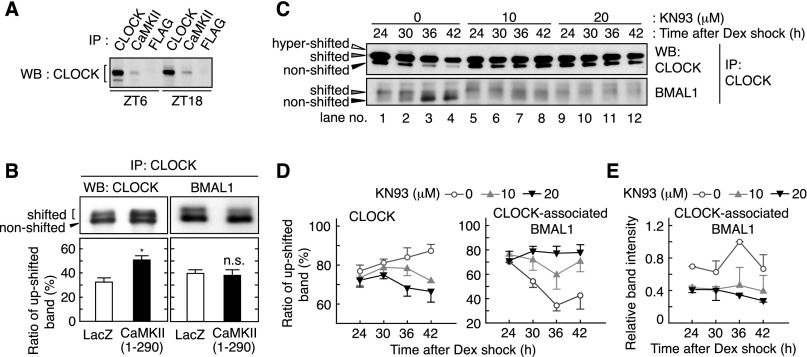
Figure 6.
CaMKII promotes phosphorylation of CLOCK and interaction of CLOCK with BMAL1. (A) Association of CLOCK and CaMKII in vivo. The lysates were prepared from the liver sampled at the indicated time points and used for immunoprecipitation by anti-CLOCK (positive control), anti-CaMKIIα/γ/δ or anti-Flag (negative control) antibody, followed by immunoblotting by anti-CLOCK antibody. (B) Effect of CaMKII overexpression on mobility shift of CLOCK and BMAL1. In HEK293 cells, CLOCK and BMAL1 were expressed with the constitutively active form of CaMKII or LacZ. The top panel shows representative raw data, and the bottom panel shows mean with SEM from three (left panel) or six (right panel) independent experiments. (*) P < 0.005 (Student’s t-test). (C) Effect of CaMKII inhibitor on heterodimers of CLOCK and BMAL1. Rat-1 cells were cultured as described in the legend for Figure 4 and sampled at the indicated time points after the rhythm induction by dexamethasone. The cell lysates were used for immunoprecipitation by anti-CLOCK antibody, and the precipitates were immunoblotted by anti-CLOCK (top panel) or anti-BMAL1 (bottom panel) antibody. (D) Ratio of the up-shifted form of CLOCK and BMAL1 under CaMKII inhibition. The ratio means the up-shifted band intensity per total band intensity of CLOCK (left panel) or BMAL1 (right panel). (E) Decreased interaction of CLOCK with BMAL1 under CaMKII inhibition. Representative data (A,C) or the mean with SEM (D,E) from three independent experiments are shown.