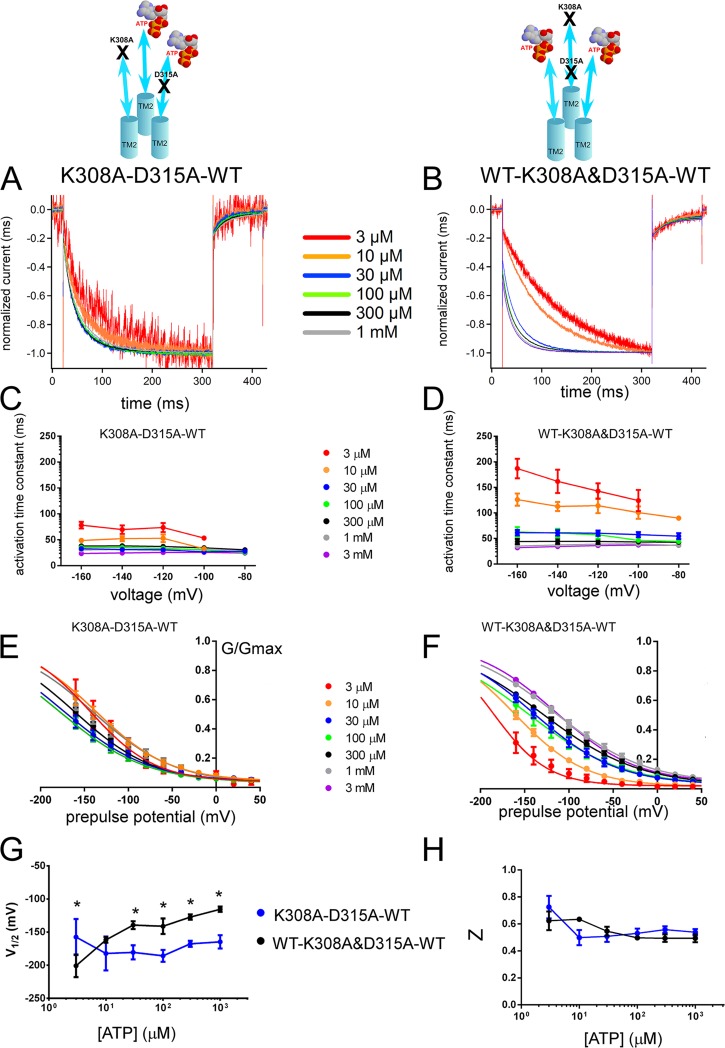
Figure 6.
Voltage- and [ATP]-dependent gating differs depending on the location of one D315A relative to the location of K308A in a trimer with two intact ATP-binding sites. Analysis of trans (K308A–D315A–WT) and cis (WT–K308A&D315A–WT) constructs shows different voltage- and [ATP]-dependent gating. (A and B) Voltage-induced normalized activation traces at −160 mV of trans (K308A–D315A–WT; A) and cis (WT–K308A&D315A–WT; B) at various [ATP] from the representative data shown in Fig. S7. (C and D) Dependence of the activation time constants on voltage and [ATP] for trans (C) and cis (D) constructs. Mean (± SEM) activation time constants for trans (C; n = 8–15) and cis (D; n = 14–19) constructs at various [ATP] and membrane potentials are shown. (E and F) Normalized G-V relationship at various [ATP] for tandem trimer trans (n = 9–20; E) and cis (n = 8–11; F) derived from the maximum tail current responses at −60 mV by fitting with the two-state Boltzmann equation, as described in Materials and methods from the same oocytes. (G and H) Mean ± SEM of V1/2 (mV) (G) and Z (H) values for the trans (n = 9–20) and cis (n = 8–11) constructs. *, P < 0.05.