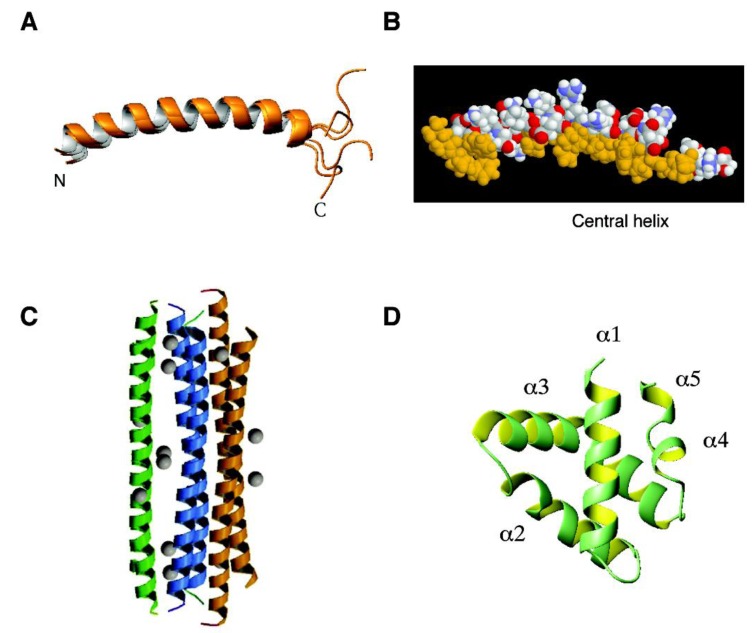
Figure 1.
Three-dimensional structures of human antimicrobial peptides from the α-helical family: (A) and (B) human cathelicidin LL-37 determined by NMR spectroscopy (PDB ID: 2K6O); (C) dermcidin determined by X-ray crystallography (PDB ID, 2YMK); and (D) granulysin determined by X-ray diffraction (PDB ID: 1L9L). In the case of LL-37, an ensemble of five structures is shown to better view the disordered C-terminal tail (A), whereas a space-filling model is given to show the segregation of the hydrophobic surface (gold) into two domains (B) [182]. The longer one corresponds to the central helix which is important for antimicrobial, anti-biofilm and antiviral activities [83]. Images were generated by using the software MOLMOL [218]. Further details can be found in the text.