Abstract
Neurons in the supplementary eye field (SEF) of the macaque monkey exhibit rank selectivity, firing differentially as a function of the phase attained during the performance of a task requiring the execution of saccades to a series of objects in fixed order. The activity of these neurons is commonly thought to represent ordinal position in the service of serial-order performance. However, there is little evidence causally linking neuronal activity in the SEF to sequential behavior. To explore the role of the SEF in serial-order performance, we delivered intracortical microstimulation while monkeys performed a task requiring them to make saccades to three objects in a fixed order on each trial. Microstimulation, considered on average across all SEF sites and all phases of the trial, affected saccadic kinematics. In particular, it prolonged the reaction time, increased the peak velocity, and slightly increased the amplitude of saccades. In addition, it interfered with the monkeys' ability to select the target appropriate to a given phase of the trial. The pattern of the errors was such as would be expected if microstimulation shifted the neural representation of ordinal position toward a later phase of the trial.
Keywords: monkey, supplementary eye field, microstimulation, serial order
it is widely thought that the supplementary eye field (SEF) is involved in serial-order performance including specifically the execution of sequences of saccades (Gaymard et al. 1998; Heide et al. 2001; Isoda and Tanji 2002, 2003; Lu et al. 2002; Pierrot-Deseilligny et al. 2002; Sommer and Tehovnik 1999; Tanji et al. 1996; Tehovnik et al. 2000). This view is consonant with the observation that the SEF contains neurons differentially active during the performance of serial-order tasks, including rank-selective neurons sensitive to the phase that the monkey has attained in a series of saccades (Berdyyeva and Olson 2009, 2010, 2011; Isoda and Tanji 2002, 2003; Lu et al. 2002).
However, direct evidence for a causal role of the SEF in serial-order performance is limited. Destruction or inactivation of the SEF interferes with the ability to perform tasks such as the double-step task that require multiple saccades (Gaymard et al. 1998; Pierrot-Deseilligny et al. 2002; Schiller and Chou 2000; Sommer and Tehovnik 1999; Tehovnik et al. 2000). Microstimulation of the SEF also interferes with performance of the double-step task (Histed and Miller 2006) by inducing an occasional reordering of the two saccades. The degradation of performance in these cases could, however, arise from impairment of several abilities not directly related to the control of sequential behavior, including the temporal discrimination of cues presented in rapid sequence, short-term memory for the timing and location of the cues, and the execution of saccades to unmarked locations.
In the present study, we examined the impact of microstimulation of the SEF on performance of a task requiring the monkey to make saccades to three objects in succession regardless of their location (Fig. 1). This task is well suited to assessing the contribution of the SEF to serial-order performance because it places minimal demands on abilities other than the ability to select objects in a required sequence. Furthermore, SEF neurons are known to carry rank-related signals in the context of this task (Berdyyeva and Olson 2009, 2010, 2011).
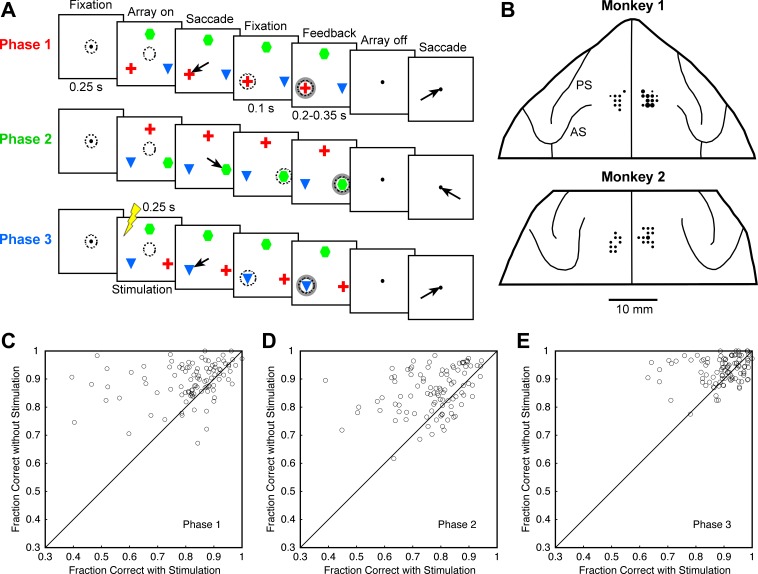
Fig. 1.
A: sequence of events in a typical trial of the serial-order task. Arrows indicate saccades, and dashed circles indicate direction of gaze. Monkeys had to make a saccade toward the red cross in the first trial phase, to the green hexagon during the second phase, and to the blue triangle during the third phase, irrespective of the objects' locations. The locations of the objects were subject to change between each trial phase and the next. Electrical stimulation (indicated during phase 3) occurred only on some trials and only during a single phase on a given trial. This might, with equal probability, be phase 1, 2, or 3. B: stimulation sites projected onto the frontal lobes in dorsal view. The area of the circle at each location is proportional to the number of sites studied during penetrations at that location (range: 1–6). PS, principal sulcus; AS, arcuate sulcus. C–E: for trial phases 1, 2, and 3, the fraction of trials on which the monkey made a saccade to the correct target rather than to either of the 2 incorrect targets. In each plot, there is 1 point for each stimulation site; x- and y-axes indicate trials on which electrical stimulation was or was not delivered during the corresponding phase, respectively.
MATERIALS AND METHODS
Data Collection
Subjects.
Two adult male rhesus macaque monkeys (Macaca mulatta) were used for this experiment (monkeys O and T designated here as monkeys 1 and 2, respectively). Experimental procedures were approved by the Carnegie Mellon University Animal Care and Use Committee and were in compliance with the guidelines set forth in the U. S. Public Health Service Guide for the Care and Use of Laboratory Animals.
Preparatory surgery.
Each monkey underwent sterile surgery under general anesthesia maintained with isoflurane inhalation. The top of the skull was exposed, bone screws were inserted around the perimeter of the exposed area, and a continuous cap of rapidly hardening acrylic was laid down so as to cover the skull and embed the heads of the screws. A head restraint bar was embedded in the cap, and scleral search coils were implanted. After initial training a 2-cm-diameter disk of skull centered on the midline of the brain roughly at anterior 21 mm (Horsley-Clarke reference frame) was removed, and a cylindrical recording chamber was cemented into the hole with its base flush to the exposed dural membrane.
Behavioral control and data collection.
All aspects of behavioral procedure, including presentation of stimuli, monitoring of eye movements, and delivery of reward, were under the control of a computer running Cortex software (NIMH Cortex) in a DOS operating system. Eye position was monitored by means of a scleral search coil system (Riverbend Instruments, Birmingham, AL). The x- and y-coordinates of eye position were stored at 4-ms intervals. Reward was delivered through a spigot under control of a solenoid valve upon successful completion of each trial.
Definition of SEF.
All stimulated sites were located within a region identified as the SEF on the basis of standard criteria (Russo and Bruce 1993, 2000). These criteria were as follows: 1) the site was located 4–8 mm rostral to the genu of the arcuate sulcus and 2–6 mm lateral to the midline as determined by structural MRI; 2) at this site or an immediately adjacent site, fixed-vector or convergent eye movements could be reproducibly elicited with microstimulation at a current strength < 80 μA (delivered in a 100-ms train of biphasic pulses of 0.2-ms duration at 333 Hz); 3) neither at this site nor at immediately adjacent sites did microstimulation at current levels up to 200 μA elicit orofacial or other bodily movements; 4) the site was at least 1 mm rostral to the supplementary motor area and 1 mm lateral to the presupplementary motor area as identified on the basis of skeletomotor responses to microstimulation (Picard and Strick 1996).
Steps of data collection.
At the beginning of each day's session, a varnish-coated tungsten microelectrode with an initial impedance of 0.5–1 MΩ at 1 kHz (Frederick Haer, Bowdoinham, ME) was advanced vertically through the dura into the underlying cortex with a hydraulic microdrive (Narashige, Tokyo, Japan). The electrode could be placed reproducibly at points forming a square grid with 1-mm spacing (Crist et al. 1988). From the point at which neural activity was first detected, we advanced the electrode ∼300 μm. Then we carried out microstimulation to determine the current threshold for eliciting eye movements in the context of a fixation task (described below) designed to maximize the likelihood of a saccade being elicited. Stimulation, delivered by a Grass S88 stimulator, consisted of 250-ms trains of 333-Hz bipolar pulses with a duration of 0.2 ms per phase and with equal cathodal and anodal amplitude. We applied currents ranging up to 200 μA (measured as the magnitude of the cathodal pulse). If we found a current level at which eye movements could be elicited consistently, then we reduced the current in steps until eye movements occurred on ∼50% of trials. We defined the level of current required to give this outcome as the fixation-task threshold. In accordance with the observations of previous authors, we found that there were sites within the limits of the SEF at which it was not possible to elicit saccades with current levels beneath 200 μA (Schlag and Schlag-Rey 1987). This was true of 18 of 101 sites in the present study. The aim of this step was to measure the current threshold. We did not systematically measure or record the metrics of the elicited saccades.
Having completed testing in the fixation task, we proceeded to examine the effect of intracortical microstimulation during performance of the serial object task (described below). We selected the current level to be used in this task according to the following principles: If the fixation-task threshold was below 100 μA, then we used this level of current in the serial object task. If the threshold was above 100 μA, or if saccadic eye movements could not be elicited with a current up to 200 μA, then we used a 100-μA current in the serial object task. This approach was based on the assumption that the minimal current required to elicit saccades consistently during the intertrial interval of the fixation task, although too low to elicit overt saccades while the monkey was engaged in voluntary control of eye position during the serial object task, would be sufficiently high to influence performance in the serial object task. This supposition was confirmed by subsequent observations as described in results. The lowest and highest currents employed in the serial object task were 25 and 100 μA.
Once testing was complete at a given site, we either advanced the electrode for ∼500 μm and repeated the procedure or selected a new grid location. In no case did we advance the electrode >2,000 μm beyond the depth at which neural activity had first been encountered. Since the type of the saccade (convergent or fixed vector), the direction or end point of the saccade, and the threshold for eliciting a saccade varied markedly with depth, we treated each position of the electrode as a distinct site.
Fixation task.
This task provided conditions maximally favorable to the occurrence of microstimulation-induced eye movements. The function of the trials themselves was to keep the monkeys alert and engaged. Microstimulation occurred during the intertrial intervals when no fixation target was present and when there was no requirement to maintain fixation. Each task began with the appearance of a 0.2° white spot that could occupy the center of the monitor or one of six locations distributed at 60° intervals around the clock at an eccentricity of 11.4°. One of these locations was directly above the center of the screen. The monkey was required to maintain fixation on the spot for a random interval in the range 200–2,000 ms, after which it was extinguished and juice was delivered. Electrical stimulation occurred on 25% of trials beginning at the instant when the spot was extinguished. This was a suitable interval in which to search for electrically elicited eye movements because, in the absence of stimulation, the eyes tended to idle on the location of the most recent spot. The next trial began after an interval of 50–250 ms.
Serial object task.
This task required monkeys to make saccades to three images (a red cross, a green hexagon, and a blue triangle) in the same order on every trial. After each saccade, the images were subject to rearrangement. Thus the monkey could not plan a fixed series of movements in advance. The monkey initiated a trial by acquiring central fixation. Two hundred and fifty milliseconds after attainment of fixation, an array containing the three objects (each ∼4.5° across) appeared at locations 11.4° eccentric spaced at equal intervals of 120° around fixation: straight up, down and to the right, and down and to the left. Appearance of the array of objects coincided with the disappearance of the central fixation spot, which signaled the monkey to make a saccade to the first object. The monkey was required to initiate saccade within 1,000 ms after disappearance of the central fixation spot. One hundred milliseconds after completion of the saccade, a feedback stimulus appeared: a white annulus centered on the object. After a variable additional interval of eccentric fixation (225–350 ms), all peripheral stimuli vanished and the central fixation spot reappeared, signaling the monkey to execute a saccade back to the center. After 150 ms of central fixation, the array of objects reappeared and the series of events was repeated with the sole exception that the saccade must be to the second object. The third phase of the trial consisted of an equivalent series of events with the third object as the target. The trial terminated with a brief 25 ms of central fixation (which ensured that the monkey returned to the center), followed by offset of all stimuli and delivery of reward. In the case of a saccade toward an incorrect object or a fixation break, the trial was aborted. The current target (red cross during phase 1, green hexagon during phase 2, and blue triangle during phase 3) could occupy any of 3 locations; therefore, there were 27 possible sequences of target locations. The two nontarget images were assigned randomly to the two nontarget locations at each phase of the trial. The target array remained on the screen during a period encompassing the saccadic reaction time (∼250 ms) and a period of postsaccadic eccentric fixation (at least 300 ms). Microstimulation began with the onset of the target array and ceased 250 ms later.
Four stimulation conditions were imposed with equal frequency on randomly interleaved trials: 1) during the first trial phase; 2) during the second trial phase; 3) during the third trial phase; 4) no microstimulation. There were 108 total conditions representing 27 sequences of target locations under each of the 4 microstimulation conditions. A run terminated when the monkey completed 2 trials successfully under each of the 108 conditions; therefore, the monkey was required to correctly perform a total of 216 trials. This design allowed full counterbalancing of trial phase against target location and the occurrence or nonoccurrence of microstimulation.
Behavioral Measures
Saccade initiation.
We counted as failures of saccade initiation those cases in which the imperative signal (offset of the central fixation spot) occurred but the eyes failed to leave the central fixation window within the allotted time of 1,000 ms.
Target attainment.
Considering cases in which the eye left the fixation window within the allotted time, we measured the frequency with which the saccade failed to land within any of the three target windows.
Percent correct.
Considering cases in which the eye landed within one of the three target windows, we measured the frequency with which the selected image was the correct one for the present phase of the trial. The computation for each phase was based on all trials completed successfully up to the point of the eyes' landing during that phase on one of the three targets regardless of success or failure later in the trial. Saccades that landed on a correct target were expressed as a proportion of saccades that landed on any target.
Reaction time.
Saccade reaction time was defined as the interval between offset of the fixation spot and the initiation of saccade. Initiation of a saccade was defined as the time of the first of the three consecutive 4-ms bins during which saccade velocity exceeded 80°/s. This measure was based exclusively on successfully completed trials. We selected this criterion as being well above the noise but well below the peak velocity of even the slowest target-directed saccades. There is a potential concern that stimulation-induced changes in saccade dynamics might contaminate the measured reaction times. However, this concern is obviated by the observation, described in results, that stimulation speeded the peak velocity whereas it retarded the reaction time.
Peak velocity.
Eye velocity was calculated bin by bin by dividing displacement of the eye position by 4 ms (duration of each bin). Peak velocity was estimated over a time interval starting from the offset of the fixation spot and lasting until the target was acquired. This measure was based exclusively on successfully completed trials.
Amplitude.
Saccade amplitude was taken as the distance in degrees of visual angle between eye positions recorded 20 ms before initiation of the saccade and 70 ms after its completion. Completion of the saccade was defined as the first of the three consecutive 4-ms bins during which saccade velocity was <20°/s measured in the time interval starting from the instant maximal speed was reached. This measure was based exclusively on successfully completed trials.
Statistical Analysis
In the case of behavioral measures based on discrete events (some saccade initiated or not; some target attained or not; the attained target correct or not), we based comparisons on a χ2-test. For example, we classified microstimulation as affecting the percent correct at a given cortical site if the observed counts of correct and incorrect choices on stimulated and nonstimulated trials deviated significantly from the counts predicted on the basis of the null hypothesis that the trials were drawn from a single parent population. Likewise, to determine whether microstimulation produced a net effect on the percent correct across all sites, we applied the same test to the counts of correct and incorrect choices summed across stimulated and nonstimulated trials at all sites. In the case of continuous measures (reaction time, peak velocity, amplitude), we based comparisons on a Wilcoxon rank sum test. For example, we classified microstimulation as affecting the reaction time at a given cortical site if the median reaction time on stimulated trials was significantly different from the median reaction time on nonstimulated trials. To determine whether microstimulation produced a net effect on reaction time across all sites, we applied the same test to the distribution of mean reaction times from all sites on stimulated and nonstimulated trials.
RESULTS
The general aim of this study was to characterize the impact of microstimulation of the SEF on performance of a serial-order task. The task required the monkey to make saccades to three objects identified by shape and color in a fixed sequence regardless of their locations, which were scrambled after each saccade (Fig. 1). An alternating current of an amplitude in the range 25–100 μA was delivered for 250 ms beginning at the time of the event signaling the monkey to make a saccade (offset of the central fixation spot and simultaneous onset of the peripheral array). The current employed at each site was below the level required for eliciting eye movements at that site in the context of the serial object task. On equal numbers of trials, microstimulation was delivered at the time of the first, second, or third saccade. On the remaining quarter of trials, which served as a control, there was no microstimulation. We documented the behavioral effects of microstimulation at 101 sites spanning both hemispheres in two monkeys (55 sites in monkey 1 and 46 sites in monkey 2). These sites (Fig. 1B) were within the confines of the SEF as determined by mapping with electrical stimulation and by post hoc measurement of their location relative to the interhemispheric midline and the genu of the arcuate sulcus (Histed and Miller 2006; Olson and Tremblay 2000).
Saccade Initiation
To determine whether microstimulation interfered with the ability to initiate a saccade, we counted cases in which the imperative signal (offset of the central fixation spot) was delivered but the eyes failed to leave the central fixation window within the allotted time (1,000 ms). In data collapsed across all sites in both monkeys and collapsed for each site across trial phases, the failure rate, although low, was higher on stimulated trials (2.3%) than on nonstimulated trials (1.4%). This represents an increase of 64%. The difference was significant (χ2-test, P < 10−10). The number of individual sites at which the difference was significant, although small (11 of 101), was significantly above the 5% expected by chance (χ2-test, P = 0.012). The fact that the net rate of initiation errors was so low precluded detailed analysis of its dependence on microstimulation site or phase of the trial. We conclude that microstimulation interfered slightly with saccade initiation.
Target Attainment
Considering cases in which the eye left the fixation window within the allotted time, we measured the frequency with which the saccade failed to land within any of the three target windows. In data collapsed across all sites in both monkeys, the rate of failure to attain any target, although small, was higher on stimulated trials (2.1%) than on nonstimulated trials (1.6%). This represents an increase of 31%. The difference was significant (χ2-test, P = 0.00006). The number of individual sites exhibiting a significant effect (7 of 101) was above the 5% expected by chance, but the difference did not approach significance (χ2-test, P = 0.77). The fact that the net rate of errors was so low precluded detailed analysis of its dependence on microstimulation site or phase of the trial. We conclude that microstimulation slightly reduced the tendency for a saccade, once launched, to land on a target.
Percent Correct
Net effect.
Considering cases in which the eye landed within one of the three target windows, we measured the frequency with which the selected image was the correct one for the present phase of the trial (Fig. 1, C–E). The computation for each phase was based on all trials completed up to the point of the eyes' landing on a target during that phase regardless of whether the target was the correct one and regardless of whether the monkey later completed the trial successfully. The mean percent correct (the average of the values from 101 individual sites) was lower on stimulated trials (81.6%) than on nonstimulated trials (89.0%). This represents a decrease of 8.3%. The difference was highly significant in the combined data (χ2-test, P < 0.0001) and remained significant in each monkey considered individually. We conclude that the net effect of microstimulation, as considered across all of the cortical sites, was to reduce the ability of the monkeys to select the correct target.
Cortical site.
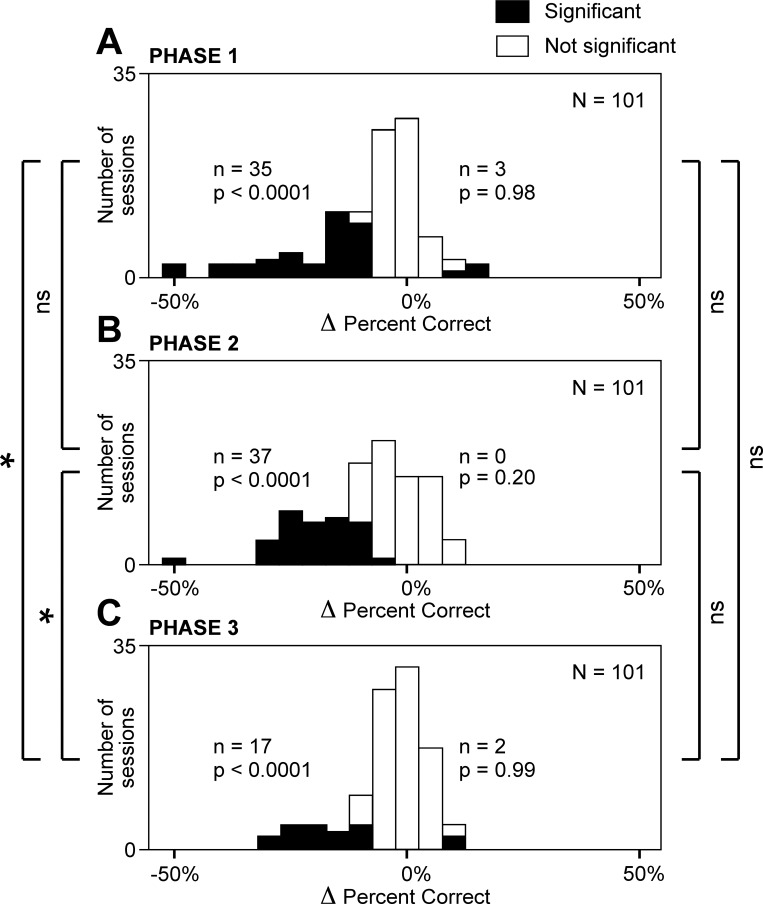
To determine whether the nature of the effect induced by microstimulation varied across cortical sites, we considered sites at which there was a significant change in percent correct (χ2-test, α = 0.05). These sites are represented by the filled portions of the frequency histograms in Fig. 2. It is evident from inspection of the histograms that most of the significant effects involved a reduction of percent correct. The number of cases of significant reduction was significantly in excess of the count expected by chance during every trial phase (χ2-test, Fig. 2). In contrast, the number of cases of significant increase did not exceed the number expected by chance during any trial phase (Fig. 2). We conclude that the uniform effect of microstimulation was to induce errors.
Fig. 2.
Microstimulation of the supplementary eye field (SEF) reduced the frequency with which the monkeys chose the target appropriate to a given phase of the trial (red cross during phase 1, green hexagon during phase 2, and blue triangle during phase 3). Each panel plots the number of cortical sites at which an effect occurred against the size of the effect. Effect size (Δ percent correct) was computed as percentage correct on trials with microstimulation minus percentage correct on trials without microstimulation. The filled sector of each bar represents sites at which the percent correct was significantly different during microstimulation from in its absence. By chance alone, increases and decreases would have been equally frequent. The n and P values on left of each panel indicate the number of sites at which microstimulation induced a significant decrease and the likelihood that this count would have been obtained by chance, respectively. The n and P values on right of each panel apply similarly to cases involving a significant increase. A: trial phase 1. B: trial phase 2. C: trial phase 3. *Difference in count between two indicated trial phases was significant (α = 0.05); ns, difference in count between 2 indicated trial phases was not significant. Symbols on far left concern cases in which microstimulation induced a significant decrease; symbols on far right concern counts of cases in which microstimulation induced a significant increase.
Trial phase.
To determine whether the effect of microstimulation varied across the three trial phases, we compared phases with respect to the number of sites at which significant effects occurred. The number of sites at which microstimulation caused a significant reduction in percent correct was significantly higher during phases 1 and 2 than during phase 3 (χ2-test, P < 0.01; Fig. 2). We conclude that microstimulation reduced the rate of correct target selection more strongly during the earlier two phases of the trial than during the third phase.
Nature of Wrong Choices
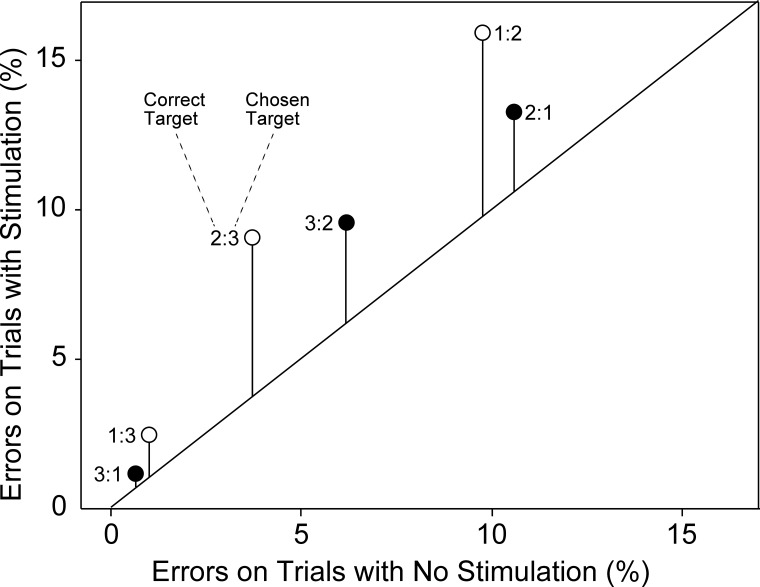
The fact that microstimulation reduced the percentage of correct choices more severely earlier in the trial could have been due to at least two effects: 1) microstimulation induced a nonspecific disruption to which behavior was more susceptible early in the trial or 2) microstimulation induced a specific bias to behave as if the trial were in a phase later than the actual one. To distinguish between these possibilities, we computed, for each phase of the trial at each site and under each condition of microstimulation, the fraction of saccades directed to each of the two available incorrect targets. Then we computed the average of each measure across all sites. The results are given in Table 1 and Fig. 3.
Table 1.
Pattern of erroneous choices
| Chosen in Phase 1 |
Chosen in Phase 2 |
Chosen in Phase 3 |
||||
|---|---|---|---|---|---|---|
| 2nd | 3rd | 1st | 3rd | 1st | 2nd | |
| No microstimulation | 9.7 | 1.0 | 10.6 | 3.7 | 0.6 | 6.2 |
| Stimulation | 16.0 | 2.5 | 13.3 | 9.1 | 1.2 | 9.6 |
| Shift | +6.3 | +1.5 | +2.8 | +5.4 | +0.5 | +3.4 |
Values are percentages of trials involving each of 6 possible errors. The analysis is based on all trials in which the monkey made a saccade to 1 of the 3 targets during a given phase of the trial. The percentage specifies the fraction of these trials in which the target chosen was inappropriate because it was associated with another phase of the trial.
Fig. 3.
Erroneous choices conformed to a systematic pattern. For each of 6 possible errors, the rate of occurrence on trials with microstimulation is plotted against the rate of occurrence on trials without microstimulation. Each point is identified by a label of the form k:j, where k indicates the phase of the trial on which the error occurred and j indicates the phase of the trial with which the target selected by the monkey was associated. The length of each vertical line segment indicates the increase in the error rate induced by microstimulation. The monkeys rarely confused targets 1 and 3, more often confused targets 2 and 3, and most often confused targets 1 and 2. In the case of each confusable pair, microstimulation enhanced choice of the later when the earlier should have been chosen more than it enhanced choice of the earlier when the later should have been chosen.
The overall pattern of errors on trials with and without microstimulation was the same: targets 1 and 3 were rarely confused, targets 2 and 3 were confused at an intermediate rate, and targets 1 and 2 were confused at a high rate. The impact of microstimulation on this pattern was not simply additive or multiplicative but rather involved a systematic distortion: the rate of choosing a target associated with a phase later than the current one (open symbols in Fig. 3) rose more than the rate of choosing a target associated with an earlier phase (filled symbols in Fig. 3). On the assumption that errors induced by microstimulation were sometimes nonsystematic (going with equal probability to the 2 wrong targets) and sometimes systematic (always going to the later wrong target), we computed the fraction of microstimulation-induced errors that were systematic, using the formula (ΔL − ΔE)/(ΔL + ΔE), where ΔL and ΔE represent the microstimulation-induced increase in later and earlier wrong choices, respectively. The value of this index was 0.039. The excess of microstimulation-induced late-target errors over microstimulation-induced early-target errors, although small, was statistically significant [P < 0.025 for all trial phases; paired t-test comparing the increase in late-target errors (ΔL) to the increase in early-target errors (ΔE) across 101 sites].
In the above analysis, there was a partial confound between the phase of the trial and the nature of the error (all errors during phase 1 necessarily involved selection of a target associated with a later trial phase, and all errors during phase 3 necessarily involved selection of a target associated with an earlier trial phase). To obviate this confound, we carried out an additional analysis, focused exclusively on phase 2, during which one of the possible wrong choices involved a target from an earlier phase of the trial (phase 1) while the other wrong choice involved a target from a later phase of the trial (phase 3). Even during this trial phase, microstimulation induced more late-target than early-target errors. The expression (ΔL − ΔE)/(ΔL + ΔE) had a value of 0.32. This is the value that would arise if the monkeys distributed their choices at random between the two distractors on 68% of trials but systematically chose the late distractor over the early one on 32% of trials. The accentuation of target 3 over target 1 choices was significant (paired t-test comparing the increase during phase 2 in target 3 choices to the increase during phase 2 in target 1 choices across 101 sites: P = 0.024). The fact that errors constituted only a small fraction of all trials precluded assessing the significance of this effect at individual sites. We conclude that SEF microstimulation increased the rate of all types of erroneous target choices but especially enhanced the tendency to choose a target associated with a trial phase later than the present one.
In the course of analyzing the nature of erroneous choices, we noted certain additional patterns that merit brief note. These involved the dependence of performance during a given trial phase (n) on spatial factors present during the immediately preceding trial phase (n − 1). We first asked whether an erroneous saccade executed during phase n tended to match or not to match in direction the correct saccade executed during phase n − 1. We found that it tended to match the preceding saccade in direction. This effect occurred only under conditions of electrical stimulation (χ2-test, P < 0.05). We next asked whether an erroneous saccade executed during phase n tended to hit or to avoid the location occupied, during phase n − 1, by the target associated with phase n. We found that it tended to avoid the prior location of the target. This effect occurred under both stimulated and nonstimulated conditions (χ2-test, P < 0.05). These effects cannot explain the tendency to err by selecting a target from a later rather than an earlier phase of the trial because the respective factors were fully counterbalanced across trials.
Dependence of Wrong Choices on Target Location
The microstimulation-induced reduction in the rate of correct choices might have involved saccades in all directions or, alternatively, might have been direction specific. To distinguish between these possibilities, we performed the following analysis. For each location of the correct object (ipsilateral, upper, or contralateral), we measured the difference in the proportion of correct saccades between stimulated trials and nonstimulated trials (Δi, Δu, Δc, with negative Δ indicating a reduction in the proportion of correct saccades on stimulated trials). In data collapsed across all sites, each Δ was negative (Δi = −0.069; Δu = −0.10; Δc = −0.094). This remained true with consideration restricted to each monkey. To assess the statistical significance of the differences among directions, we computed Δi, Δu, and Δc separately for each of the 101 sites. Upon comparing the distributions, we found that the effect of microstimulation was significantly less for ipsilateral than for upper or contralateral targets (Wilcoxon signed-rank test, |Δi| < |Δu|, P = 0.008; |Δi| < |Δc|, P = 0.05; Δu not significantly different from Δc, P = 0.78). The fact that errors constituted only a small fraction of all trials precluded assessing the significance of this effect at individual sites.
Reaction Time
Net effect.
Considering all successfully completed trials, we measured the reaction time to initiate a saccade during each trial phase. The mean reaction time (the average of the medians from 101 individual sites) was longer on stimulated trials (276 ms) than on nonstimulated trials (250 ms). This represents an increase of 10%. The difference was highly significant in the combined data (Wilcoxon rank sum test, P = 0.004) and remained significant in each monkey considered individually. We conclude that the overall effect of microstimulation was to prolong the reaction time.
Cortical site.
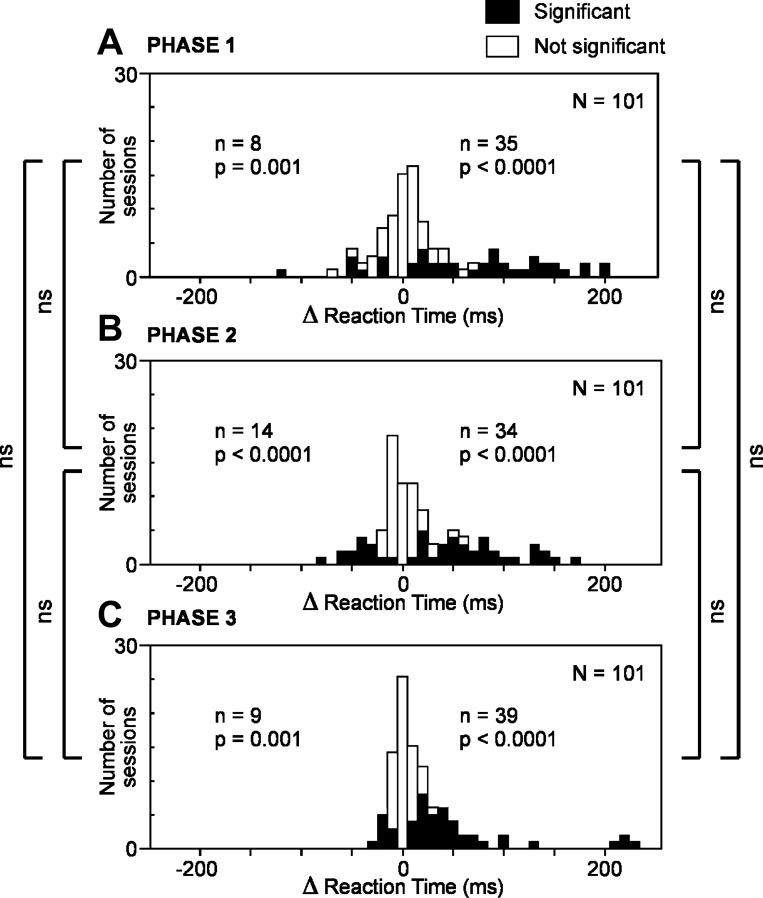
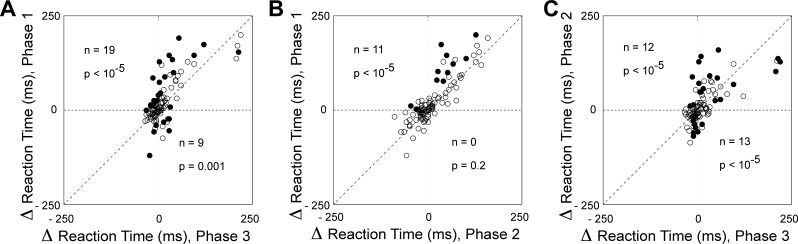
To determine whether microstimulation could, at individual sites, shorten as well as prolong the reaction time, we counted cases involving a significant change in reaction time during a given phase (Fig. 4). The number of cases of significant prolongation [delta reaction time (Δrt) > 0, χ2-test, α = 0.05] was significantly in excess of the count expected by chance (2.5%) during each trial phase (χ2-test, P < 0.0001). The number of cases of significant shortening (Δrt < 0, χ2-test, α = 0.05) also exceeded chance expectation during each trial phase (χ2-test, P < 0.001). We conclude that there was a genuine cross-site difference in the impact of microstimulation, with microstimulation at some sites (a majority) prolonging the reaction time and microstimulation at other sites (a minority) shortening it.
Fig. 4.
Microstimulation of the SEF produced an increase in saccadic reaction time at many cortical sites and a decrease in saccadic reaction time at a few sites. This was true across all trial phases. A: phase 1. B: phase 2. C: phase 3. Conventions as in Fig. 2.
Trial phase.
To determine whether the effect of microstimulation varied significantly across the phases of the trial, we compared phases with respect to the number of significant effects observed (Fig. 4). The number of sites at which microstimulation produced a significant prolongation of the reaction time (Wilcoxon rank sum test, α = 0.05) did not differ significantly across phases (χ2-test, P > 0.05) nor did the number of sites at which microstimulation produced a significant shortening of the reaction time (χ2-test, P > 0.05). We conclude that the impact of microstimulation on reaction time did not vary in a systematic pattern across trial phases.
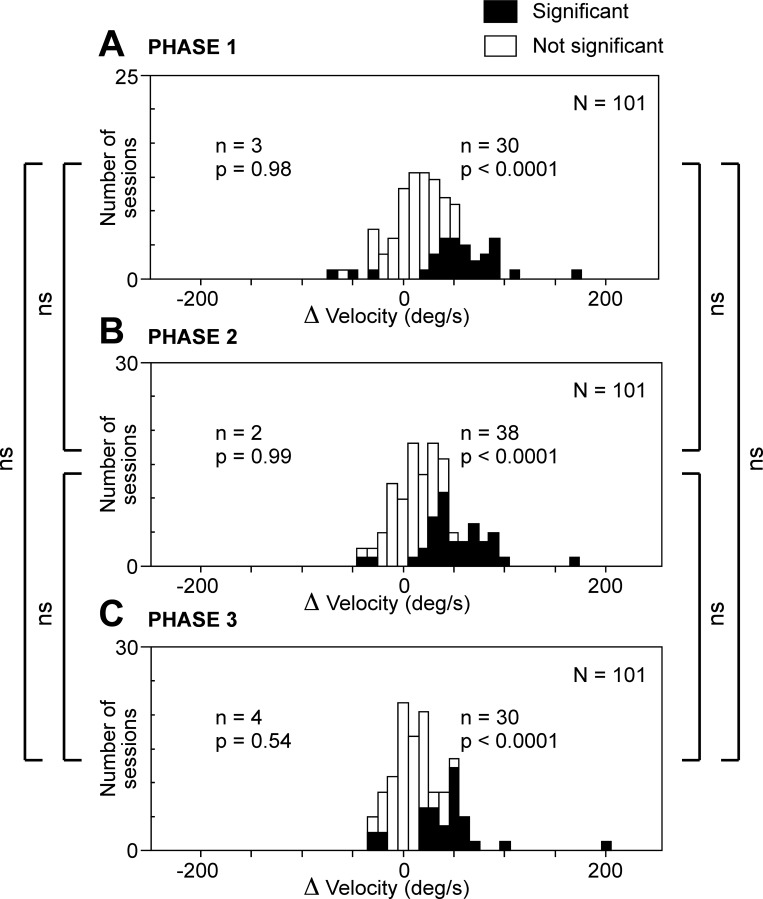
Peak Velocity
Net effect.
Considering all successfully completed trials, we measured the peak velocity of the saccade executed during each phase. The mean peak velocity (the average of the medians from 101 individual sites) was higher on stimulated trials (491°/s) than on nonstimulated trials (467°/s). This represents an increase of 5.1%. The difference was highly significant in the combined data (Wilcoxon rank sum test, P < 10−12) and remained significant in each monkey considered individually. We conclude that the overall effect of microstimulation was to increase the peak velocity of the saccade.
Cortical site.
To determine whether microstimulation could, at individual sites, decrease as well as increase peak velocity, we counted cases involving a significant change in peak velocity during a given trial phase (Fig. 5). The number of cases of significant increase [delta peak velocity (Δvel) > 0, χ2-test, α = 0.05] was significantly in excess of the count expected by chance (2.5%) during each trial phase (χ2-test, P < 0.0001). The number of cases of significant decrease [(Δvel) < 0, χ2-test, α = 0.05] was not. Thus the few cases with apparently significant decrease can be regarded as the spurious consequence of type I errors. We conclude that the impact of microstimulation was consistent across sites. If there was an effect, it took the form of an increase in peak velocity.
Fig. 5.
Microstimulation of the SEF produced an increase in peak saccadic velocity. This was true across all trial phases. A: phase 1. B: phase 2. C: phase 3. Conventions as in Fig. 2.
Trial phase.
To determine whether the effect of microstimulation varied significantly across the phases of the trial, we compared phases with respect to the number of significant effects observed (Fig. 5). The number of sites at which microstimulation caused a significant increase in peak velocity (Wilcoxon rank sum test, α = 0.05) did not differ significantly across trial phases (χ2-test, P > 0.05). We conclude that the impact of microstimulation on peak velocity was consistent across trial phases.
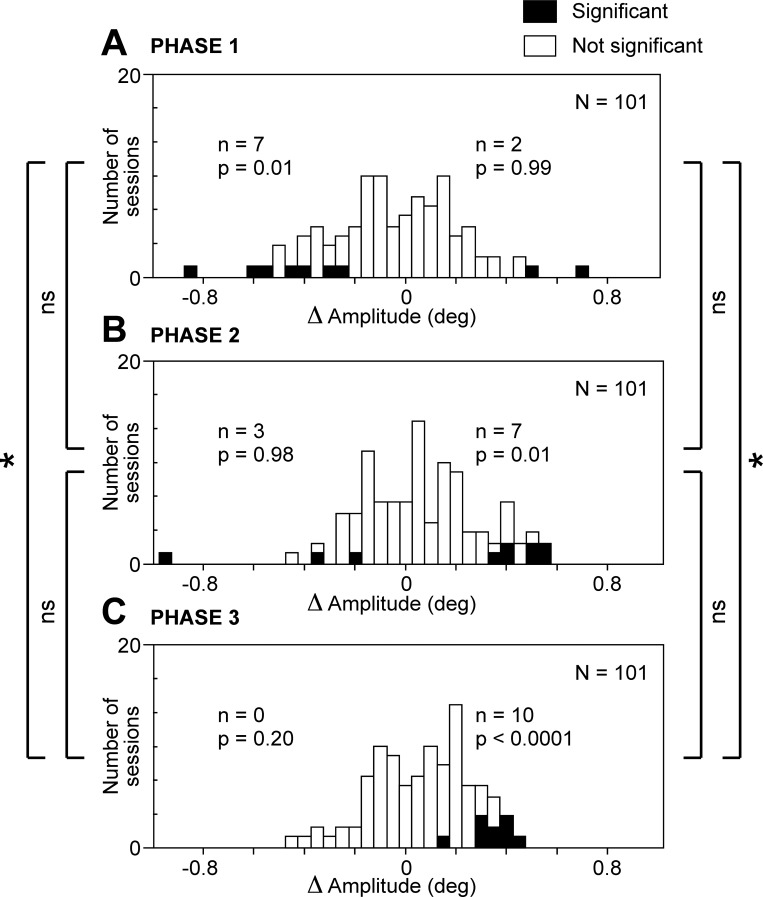
Amplitude
Net effect.
Considering all successfully completed trials, we measured the amplitude of the saccade executed during each phase. The mean saccadic amplitude (the average of the medians from 101 individual sites) was very slightly greater on stimulated trials (10.25°) than on nonstimulated trials (10.24°). This represents an increase of 0.1%. The difference achieved significance in the combined data (Wilcoxon rank sum test, P = 0.011) but was present and achieved significance only in monkey 1 (P < 0.0001). In monkey 2, the trend was opposite and did not achieve significance (P = 0.97). We conclude that the overall effect of microstimulation was a marginal increase in the amplitude of the saccades.
Cortical site.
To determine whether microstimulation at individual sites could induce a decrease as well as an increase of saccadic amplitude, we counted cases involving a significant change in amplitude during a given trial phase (Fig. 6). The number of cases of significant increase [delta amplitude (Δamp) > 0, χ2-test, α = 0.05] was significantly in excess of the count expected by chance (2.5%) during phase 2 (χ2-test, P < 0.01) and phase 3 (χ2-test, P < 0.0001). The number of cases of significant decrease (Δamp < 0, χ2-test, α = 0.05) was significantly in excess of the count expected by chance (2.5%) during phase 1 (χ2-test, P < 0.01). Thus, during each phase of the trial, the impact of microstimulation took just one form: a decrease in amplitude during phase 1 and an increase during phases 2 and 3.
Fig. 6.
Microstimulation of the SEF produced a slight decrease in saccadic amplitude during trial phase 1 and slight increases during trial phases 2 and 3. A: phase 1. B: phase 2. C: phase 3. Conventions as in Fig. 2.
Trial phase.
To assess the significance of the variation in the effect of microstimulation across the phases of the trial, we compared phases with respect to the number of significant effects observed (Fig. 6). The number of sites at which microstimulation caused a significant increase in amplitude (Wilcoxon rank sum test, α = 0.05) was significantly greater during phase 3 than during phase 1 (χ2-test, P < 0.05). The number of sites at which microstimulation caused a significant decrease in amplitude (Wilcoxon rank sum test, α = 0.05) was significantly greater during phase 1 than during phase 3 (χ2-test, P < 0.05). We conclude that there was a systematic pattern in which microstimulation tended to reduce saccadic amplitude early in the trial and increase it later.
Variation Across Cortical Sites: Behavioral Effect
We wondered whether the various behavioral effects of microstimulation were yoked in the sense that if one were strong at a given cortical site then others would be as well. To address this issue, we analyzed the correlation, across cortical sites, between each pair of measures in the following set: delta percent correct (Δpc), Δrt, Δvel, and Δamp. For each measure at each site, we computed the average value across all three task phases. We inverted the sign of Δpc so as to ensure that for each measure the predominant effect as observed across all sites would have a positive sign. Then we carried out the six possible correlation analyses (Table 2). These revealed that Δpc was positively correlated with Δrt and Δvel, in agreement with the surmise that the strengths of the various effects varied in tandem across cortical sites. However, Δrt and Δvel were negatively correlated with Δamp. in contradiction to the surmise.
Table 2.
Correlated variation of measures across cortical sites
| Correlation Coefficient (r) | Level of Significance (P) | |
|---|---|---|
| −Δ Percent correct vs. Δ reaction time | 0.66 | <0.0001 |
| −Δ Percent correct vs. Δ velocity | 0.60 | <0.0001 |
| Δ Velocity vs. Δ amplitude | −0.39 | <0.0001 |
| Δ Reaction time vs. Δ amplitude | −0.20 | 0.046 |
| −Δ Percent correct vs. Δ amplitude | 0.07 | 0.46 |
| Δ Reaction time vs. Δ velocity | 0.03 | 0.37 |
Values are results of a correlation analysis assessing the tendency for different effects of microstimulation to vary in parallel across the 101 cortical sites. The sign of the induced change in percent correct (Δ Percent correct) is inverted so as to ensure that for this, as for the other measures, an induced change corresponding to the predominant effect, reflected in the average across all cortical sites, would be positive.
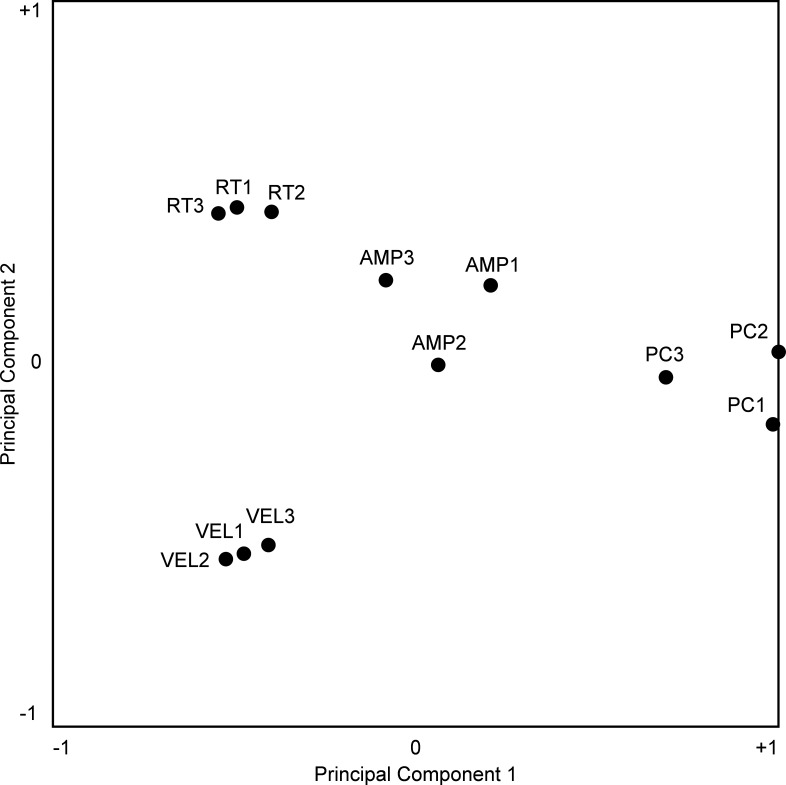
The existence of positive pairwise correlations among three of the measures does not in itself indicate that they were fully yoked. Residual variance, indicated by a correlation coefficient < 1, might simply have been noise or, alternatively, might have arisen from a genuine tendency for the measures to vary independently across cortical sites. To distinguish between these interpretations, we carried out a further analysis. We compiled a database consisting of 12 indexes computed for each of 101 sites. These represented four measures of behavior (Δpc, Δrt, Δvel, and Δamp) crossed with three trial phases (1, 2, and 3). So as to ensure that each measure contributed equally to the analysis, we normalized each raw value to the distribution of raw values obtained for that measure across all 101 sites, subtracting out the mean and dividing by the standard deviation. So as to ensure that for each index a positive value indicated an effect in the direction that predominated across all sites, we inverted the sign of each Δpc measure. We then represented the indexes as 12 points in a 101-dimensional site-space, with the location of each point relative to a given site-axis determined by the value of the index at that site. Upon carrying out a principal components analysis and projecting the points onto the plane defined by the first and second principal components (which accounted for 46% of the total variance), we found that their arrangement was highly systematic. Measures of a given behavior obtained during the three trial phases cluster together but do not overlap perfectly (Fig. 7). The small amount of scatter within each such triad might have arisen from genuine differences across epochs (with the measure greatest during phase 1 at some sites, during phase 2 at other sites, and so on) or might just have been the product of noise in the data. By either interpretation, it represents an upper bound on the amount of scatter to be expected from noise. Against this benchmark, it is evident that the triads representing different measures are more widely scattered than predicted from noise alone. The tendency for between-triad variance (mean across 101 sites: 0.058) to exceed within-triad variance (mean across 101 sites: 0.018) was highly significant (2-sample F test for equal variances, P < 0.001). We conclude that the tendency for microstimulation to affect the percent correct, the reaction time, the peak velocity, and the amplitude of the saccade was subject to a degree of independent variation across cortical sites.
Fig. 7.
Twelve measures of the change in behavior induced by electrical stimulation are projected from a space with 1 dimension for each of the 101 stimulation sites onto a plane containing the first and second principal components (PCs) of the distribution. Closer proximity between points roughly indicates a greater tendency for the measures to covary positively across sites. However, the positioning of the points is not a definitive indicator of the magnitude or sign of the correlation. For the results of an explicit correlation analysis, see Table 2. Noteworthy in this figure is the fact that points representing different effects [percent correct (ΔPC), reaction time (ΔRT), velocity (ΔVEL), and amplitude (ΔAMP)] are more widely separated than points representing the same effect as measured during successive trial phases (e.g., ΔPC1, ΔPC2, and ΔPC3).
Variation across Cortical Sites: Trial Phase
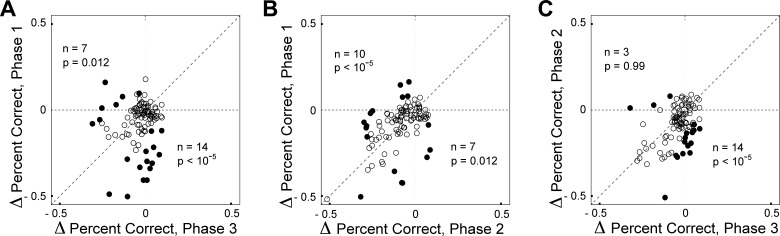
We wondered whether microstimulation had exerted its strongest effect during different trial phases at different sites. To address this issue, we carried out an additional phase of analysis. We describe the approach as applied to measures of percent correct and then summarize results obtained when it was applied to other behavioral measures. We considered each possible pair of trial phases (1 and 3, 1 and 2, and 2 and 3). For each pair, we wished to know whether there were some sites at which the reduction in percent correct was greater during the earlier phase of the pair and other sites at which it was greater during the later phase of the pair. We first carried out a χ2-test (α = 0.05) on data from each site, assessing whether the fractional change in percent correct induced by microstimulation was significantly different between the two phases of the pair. Sites represented by filled symbols in Fig. 8 are those at which the microstimulation-induced change in percent correct was significantly different between the two trial phases. Then we counted cases in which the reduction was significantly greater during the earlier phase of the pair (filled symbols below the diagonal in Fig. 8) and cases in which the reduction was significantly greater during the later phase of the pair (filled symbols above the diagonal in Fig. 8). Finally, we carried out a χ2-test (α = 0.05/3) to determine whether each count was significantly greater than the count expected by chance (2.5% of 101 sites). For phases 1 and 3 (Fig. 8A) and for phases 1 and 2 (Fig. 8B), both counts were significantly above chance. A parallel analysis of reaction time data yielded a comparable result. In particular, some sites exhibited the strongest effect during trial phase 3 while other sites exhibited the strongest effect during early trial phases 1 and 2 (Fig. 9). Parallel analyses focused on saccadic peak velocity and amplitude revealed no such trend. We conclude that cortical sites genuinely differed with regard to the phase of the trial during which microstimulation induced the strongest effects on percent correct and reaction time. The effects were strongest early in the trial at some sites and late in the trial at others.
Fig. 8.
The impact of microstimulation on percent correct was greatest during relatively early trial phases at some sites and during relatively late trial phases at other sites. A: the fractional change in percent correct induced by microstimulation during trial phase 1 is plotted against the fractional change in percent correct induced by microstimulation during trial phase 3. Fractional change = (PCs − PCn)/PCn, where PCs = percent correct on trials with microstimulation and PCn = percent correct on trials without microstimulation. Among 101 sites, there were 14 where the induced decrease was significantly greater during phase 1 and 7 where the induced decrease was significantly greater during phase 3. Both counts were significantly greater than expected by chance, as indicated by the P values. B: the fractional change in percent correct induced by microstimulation during trial phase 1 is plotted against the fractional change in percent correct induced by microstimulation during trial phase 2. C: the fractional change in percent correct induced by microstimulation during trial phase 2 is plotted against the fractional change in percent correct induced by microstimulation during trial phase 3.
Fig. 9.
The impact of microstimulation on the saccadic reaction time was greatest during relatively early trial phases at some sites and during relatively late trial phases at other sites. A: phase 1 vs. phase 3. B: phase 1 vs. phase 2. C: phase 2 vs. phase 3. All conventions as in Fig. 8.
DISCUSSION
The goal of this study was to test the idea that the SEF contributes to the control of serial-order saccadic performance. To test this idea, we measured the impact on serial-order performance of intracortical microstimulation delivered at current levels below threshold for producing an overt saccade. Microstimulation affected performance in a variety of ways summarized in Table 3. The effects fall into two general categories that we discuss separately below: alteration of the saccades themselves and interference with serial-order performance.
Table 3.
Key observations
| Saccadic Measure | Net Effect | Varied Across Cortical Sites? | Varied Across Trial Phases? | Dependence on Phase Varied Across Sites? |
|---|---|---|---|---|
| Initiation | −0.9% | — | — | — |
| Attainment | −0.5% | — | — | — |
| Percent correct | −7.4% | No | Yes | Yes |
| Reaction time | +26 ms | Yes | No | Yes |
| Peak velocity | +24°/s | No | No | No |
| Amplitude | +0.01° | No | Yes | No |
Columns display observations for behavioral measure. Net effect, mean microstimulation-induced change in each measure as computed across all cortical sites and all trial phases. The third column shows answers to the question of whether sites at which microstimulation induced a significant increase and sites at which it induced a significant decrease were both more common than expected by chance. The fourth column shows answers to the question of whether the number of sites exhibiting a significant increase or the number of sites exhibiting a significant decrease varied significantly across trial phases. The fifth column shows answers to the question of whether sites at which microstimulation produced a significantly stronger effect late in the trial and sites at which microstimulation produced a significantly stronger effect early in the trial were both more common than expected by chance.
Saccadic Metrics
Although our primary interest was in the serial aspects of behavior, we also examined the impact of microstimulation on saccade kinematics. We found that the effects of microstimulation on saccade kinematics were small but statistically significant. On average, across all cortical sites and all phases of the trial, microstimulation slightly impaired the ability of the monkeys to initiate a saccade and to direct it to one of the targets. Moreover, it affected the metrics even of successfully initiated and directed saccades. Reaction time was prolonged, peak velocity was enhanced, and amplitude was slightly increased. These distinct effects tended to vary in unison across cortical sites, as if microstimulation at some sites were more effective than at others, but they were not fully yoked. The variation from site to site might have depended on the tangential location of the stimulating electrode or, alternatively, on its laminar depth.
These findings are in general agreement with previous reports concerning the impact of SEF microstimulation on saccadic behavior. The most consistently reported effect is an increase in saccadic reaction time manifest, at the extreme, as an arrest of self-initiated saccades (Mann et al. 1988; Stuphorn and Schall 2006; Sumner et al. 2007; Yang et al. 2008; but see also Schiller and Tehovnik 2005). The magnitude of the reaction time increment noted in our study is commensurate with the magnitude of the effect as described previously (Stuphorn and Schall 2006). Previous reports have indicated that microstimulation induces no effect on peak velocity or amplitude (Histed and Miller 2006; Stuphorn and Schall 2006; Yang et al. 2008) or only a marginal effect (Kunimatsu and Tanaka 2012). Inasmuch as the effects that we observed, although statistically significant, were small, it is not surprising that they should have emerged only inconsistently in previous experiments. These observations suggest that the SEF plays a genuine role not only in the hierarchically elevated processes governing sequential behavior (Histed and Miller 2006) but also in the low-order processes governing saccade metrics.
The fact that microstimulation of the SEF prolongs the saccadic reaction time is subject to at least two interpretations. On one hand, the effect of microstimulation might be to disrupt, by the addition of noise, neuronal activity representing the action plan (Churchland and Shenoy 2007). On the other hand, microstimulation, by increasing the net level of activity in the SEF (Tehovnik et al. 2006), might render more potent a suppressive influence that it exerts on other areas directly mediating saccadic planning and execution. In support of the latter interpretation, several studies have demonstrated that neurons in the SEF fire more strongly when it is necessary to suppress prepotent responses (Amador et al. 2004; Nakamura et al. 2005; Olson and Gettner 2002; Schlag-Rey et al. 1997; Stuphorn et al. 2000). Moreover, it has been shown that patients with SEF lesions are impaired at making eye movements that violate a previous intention or an ingrained behavioral set (Husain et al. 2003; Kennard et al. 2005; Parton et al. 2007; Sumner et al. 2007).
Interference with Serial-Order Performance
On average, across all cortical sites and all phases of the trial, microstimulation significantly reduced the ability of the monkeys to select the correct target. This finding is consonant with earlier observations to the effect that microstimulation of the SEF in monkeys perturbs the order of saccades in a memory-guided double-step task (Histed and Miller 2006). It is also consistent with reports that lidocaine inactivation (Sommer and Tehovnik 1999) and lesions of the SEF (Schiller and Chou 2000) interfere with double-step performance. The impact of these manipulations on double-step performance might have arisen because integrity of the SEF is necessary for executing two saccades in proper order but might also have arisen because other aspects of the task require mediation by the SEF. We note, in particular, the requirement to form a double-step plan in response to a display presented at the beginning of the trial. This qualification applies not only to monkey studies involving the double-step task but also to human experiments assessing double-step behavior after lesions (Gaymard et al. 1990, 1993; Parton et al. 2007) or transcranial magnetic microstimulation of the SEF (Muri et al. 1994, 1995; Rosenthal et al. 2008; Tobler and Muri 2002). Our demonstration of a performance deficit in a serial-order task involving an overlearned sequence avoids this confound.
The use of a task with multiple phases spread out over time also allowed us to analyze the pattern of errors induced by microstimulation more finely than is possible in the double-step framework. We found that the error pattern induced by SEF microstimulation was not random. The monkeys made more errors during trial phases 1 and 2 than during trial phase 3 (Fig. 2). This finding suggests that microstimulation of the SEF shifted the monkeys' sense of ordinal position, causing them to behave as if in a late phase of the trial, in accordance with the observation that a majority of rank-selective neurons in the SEF prefer trial phase 3 (Berdyyeva and Olson 2009, 2010, 2011). The reduction of errors with progress through the trial might arguably have arisen from a tendency for the monkeys to become less distractible as reward approached (Sohn and Lee 2006; Tehovnik et al. 1999). However, in this framework, it is difficult to explain the existence of a few sites at which microstimulation induced more errors later in the trial (Fig. 8). The existence of such sites is compatible with the observation that a minority of rank-selective SEF neurons prefer trial phase 1 (Berdyyeva and Olson 2009, 2010, 2011).
Serial-order performance in humans can be explained by competitive queuing models in which there is no explicit representation of ordinal position (Rhodes et al. 2004). In these models, at the outset of a sequence, all of the required actions are represented in parallel but the strength of the representations is graded according to a primacy rule. The action with the strongest representation is performed first, whereupon its representation is deleted. Then the action with the next strongest representation is performed and so on. In this framework, it is straightforward to explain anticipatory substitution errors. If the representation of the currently required action is weakened, then the next action in the sequence will tend to be substituted because, out of all the remaining actions, it is the one most strongly represented. On the assumption that microstimulation of the SEF weakens the representation of the currently required action, this mechanism could explain the tendency for errors induced by stimulation to involve selection of a target later in the sequence rather than earlier. The main weakness of this account is that it provides no explanation for the existence in the SEF of neurons that do apparently encode ordinal position explicitly (Berdyyeva and Olson 2009, 2010, 2011; Isoda and Tanji 2002, 2003; Lu et al. 2002).
GRANTS
This work received support from National Institutes of Health (NIH) Grants RO1 EY-018620 and P50 MH-45156 and technical support from NIH Grants P30 EY-08098 and P41 RR-03631.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.K.B. and C.R.O. conception and design of research; T.K.B. performed experiments; T.K.B. and C.R.O. analyzed data; T.K.B. and C.R.O. interpreted results of experiments; T.K.B. and C.R.O. prepared figures; T.K.B. and C.R.O. drafted manuscript; T.K.B. and C.R.O. edited and revised manuscript; T.K.B. and C.R.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karen McCracken for excellent technical assistance.
REFERENCES
- Amador N, Schlag-Rey M, Schlag J. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. J Neurophysiol 91: 1672–1689, 2004 [DOI] [PubMed] [Google Scholar]
- Berdyyeva TK, Olson CR. Monkey supplementary eye field neurons signal the ordinal position of both actions and objects. J Neurosci 29: 591–599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyyeva TK, Olson CR. Rank signals in four areas of macaque frontal cortex during selection of actions and objects in serial order. J Neurophysiol 104: 141–159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyyeva TK, Olson CR. Relation of ordinal position signals to the expectation of reward and passage of time in four areas of the macaque frontal cortex. J Neurophysiol 105: 2547–2559, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Shenoy KV. Delay of movement caused by disruption of cortical preparatory activity. J Neurophysiol 97: 348–359, 2007 [DOI] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988 [DOI] [PubMed] [Google Scholar]
- Gaymard B, Pierrot-Deseilligny C, Rivaud S. Impairment of sequences of memory-guided saccades after supplementary motor area lesions. Ann Neurol 28: 622–626, 1990 [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Exp Brain Res 123: 159–163, 1998 [DOI] [PubMed] [Google Scholar]
- Gaymard B, Rivaud S, Pierrot-Deseilligny C. Role of the left and right supplementary motor areas in memory-guided saccade sequences. Ann Neurol 34: 404–406, 1993 [DOI] [PubMed] [Google Scholar]
- Heide W, Binkofski F, Seitz RJ, Posse S, Nitschke MF, Freund HJ, Kompf D. Activation of frontoparietal cortices during memorized triple-step sequences of saccadic eye movements: an fMRI study. Eur J Neurosci 13: 1177–1189, 2001 [DOI] [PubMed] [Google Scholar]
- Histed MH, Miller EK. Microstimulation of frontal cortex can reorder a remembered spatial sequence. PLoS Biol 4: e134, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Parton A, Hodgson TL, Mort D, Rees G. Self-control during response conflict by human supplementary eye field. Nat Neurosci 6: 117–118, 2003 [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Cellular activity in the supplementary eye field during sequential performance of multiple saccades. J Neurophysiol 88: 3541–3545, 2002 [DOI] [PubMed] [Google Scholar]
- Isoda M, Tanji J. Contrasting neuronal activity in the supplementary and frontal eye fields during temporal organization of multiple saccades. J Neurophysiol 90: 3054–3065, 2003 [DOI] [PubMed] [Google Scholar]
- Kennard C, Mannan SK, Nachev P, Parton A, Mort DJ, Rees G, Hodgson TL, Husain M. Cognitive processes in saccade generation. Ann NY Acad Sci 1039: 176–183, 2005 [DOI] [PubMed] [Google Scholar]
- Kunimatsu J, Tanaka M. Alteration of the timing of self-initiated but not reactive saccades by electrical stimulation in the supplementary eye field. Eur J Neurosci 36: 3258–3268, 2012 [DOI] [PubMed] [Google Scholar]
- Lu X, Matsuzawa M, Hikosaka O. A neural correlate of oculomotor sequences in supplementary eye field. Neuron 34: 317–325, 2002 [DOI] [PubMed] [Google Scholar]
- Mann SE, Thau R, Schiller PH. Conditional task-related responses in monkey dorsomedial frontal cortex. Exp Brain Res 69: 460–468, 1988 [DOI] [PubMed] [Google Scholar]
- Muri RM, Rivaud S, Vermersch AI, Leger JM, Pierrot-Deseilligny C. Effects of transcranial magnetic stimulation over the region of the supplementary motor area during sequences of memory-guided saccades. Exp Brain Res 104: 163–166, 1995 [DOI] [PubMed] [Google Scholar]
- Muri RM, Rosler KM, Hess CW. Influence of transcranial magnetic stimulation on the execution of memorised sequences of saccades in man. Exp Brain Res 101: 521–524, 1994 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Roesch MR, Olson CR. Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol 93: 884–908, 2005 [DOI] [PubMed] [Google Scholar]
- Olson CR, Gettner SN. Neuronal activity related to rule and conflict in macaque supplementary eye field. Physiol Behav 77: 663–670, 2002 [DOI] [PubMed] [Google Scholar]
- Olson CR, Tremblay L. Macaque supplementary eye field neurons encode object-centered locations relative to both continuous and discontinuous objects. J Neurophysiol 83: 2392–2411, 2000 [DOI] [PubMed] [Google Scholar]
- Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, Morgan PS, Jackson S, Rees G, Husain M. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia 45: 997–1008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342–353, 1996 [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Ploner CJ, Muri RM, Gaymard B, Rivaud-Pechoux S. Effects of cortical lesions on saccadic eye movements in humans. Ann NY Acad Sci 956: 216–229, 2002 [DOI] [PubMed] [Google Scholar]
- Rhodes BJ, Bullock D, Verwey WB, Averbeck BB, Page MP. Learning and production of movement sequences: behavioral, neurophysiological, and modeling perspectives. Hum Mov Sci 23: 699–746, 2004 [DOI] [PubMed] [Google Scholar]
- Rosenthal CR, Hodgson TL, Husain M, Kennard C. Supplementary eye field contributions to the execution of saccades to remembered target locations Prog Brain Res 171: 419–423, 2008 [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Effect of eye position within the orbit on electrically elicited saccadic eye movements: a comparison of the macaque monkey's frontal and supplementary eye fields. J Neurophysiol 69: 800–818, 1993 [DOI] [PubMed] [Google Scholar]
- Russo GS, Bruce CJ. Supplementary eye field: representation of saccades and relationship between neural response fields and elicited eye movements J Neurophysiol 84: 2605–2621, 2000 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Chou I. The effects of anterior arcuate and dorsomedial frontal cortex lesions on visually guided eye movements. 2. Paired and multiple targets. Vision Res 40: 1627–1638, 2000 [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Neural mechanisms underlying target selection with saccadic eye movements. Prog Brain Res 149: 157–171, 2005 [DOI] [PubMed] [Google Scholar]
- Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol 57: 179–200, 1987 [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390: 398–401, 1997 [DOI] [PubMed] [Google Scholar]
- Sohn JW, Lee D. Effects of reward expectancy on sequential eye movements in monkeys. Neural Netw 19: 1181–1191, 2006 [DOI] [PubMed] [Google Scholar]
- Sommer MA, Tehovnik EJ. Reversible inactivation of macaque dorsomedial frontal cortex: effects on saccades and fixations. Exp Brain Res 124: 429–446, 1999 [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci 9: 925–931, 2006 [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature 408: 857–860, 2000 [DOI] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54: 697–711, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J, Shima K, Mushiake H. Multiple cortical motor areas and temporal sequencing of movements. Brain Res 5: 117–122, 1996 [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Slocum WM, Schiller PH. Behavioural conditions affecting saccadic eye movements elicited electrically from the frontal lobes of primates. Eur J Neurosci 11: 2431–2443, 1999 [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev 32: 413–448, 2000 [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol 96: 512–521, 2006 [DOI] [PubMed] [Google Scholar]
- Tobler PN, Muri RM. Role of human frontal and supplementary eye fields in double step saccades. Neuroreport 13: 253–255, 2002 [DOI] [PubMed] [Google Scholar]
- Yang SN, Heinen SJ, Missal M. The effects of microstimulation of the dorsomedial frontal cortex on saccade latency. J Neurophysiol 99: 1857–1870, 2008 [DOI] [PubMed] [Google Scholar]