SUMMARY
The purpose of our study was to test the efficacy and toxicity of hyperthermia in conjunction with chemoradiotherapy for T3N0 laryngeal cancer. From 1997-2006, 25 patients diagnosed with T3N0 laryngeal carcinoma who denied laryngectomy were selected for this retrospective study. Patients received a total dose of 70 Gy (2 Gy per fraction, 5 days per week) in combination with 6 weekly sessions of hyperthermia, in addition to weekly cisplatin chemotherapy. The hyperthermia device was operated as a 433 MHz microwave heating with water loaded and water-cooled waveguides. The temperature was monitored subcutaneously in the skin under the aperture of the waveguide. The median follow-up was 60 months, while 23 of 25 patients (92%) presented complete response to treatment. The two patients that did not respond to thermoradiotherapy underwent total laryngectomy, and during follow-up were alive and free of disease. According to EORTC/RTOG criteria, toxicity was mild: three patients (12%) presented grade III, eight (32%) presented grade II and 14 (56%) presented grade I acute skin toxicity. Grade III laryngeal late toxicity (vocal cord malfunction due to severe oedema) was noted in two patients (8%) at 6-8 months post-thermo-chemoradiotherapy. Tmin was correlated (Spearman rho, p < 0.05) with response to treatment as well as with acute skin toxicity and laryngeal function. When a patient with T3N0 laryngeal carcinoma denies laryngectomy, an alternative treatment is combined thermo-chemoradiotherapy which seems to be effective and generally tolerable with radiation-induced skin toxicity and/or late side effects. A larger patient cohort is needed to confirm these results.
KEY WORDS: Hyperthermia, Laryngeal cancer, Radio-chemotherapy, Toxicity, Follow-up
RIASSUNTO
Col presente lavoro abbiamo voluto testare l'efficacia e la tossicità del trattamento combinato dei carcinomi laringei T3N0 mediante ipertermia e radio-chemioterapia. Per questo studio retrospettivo abbiamo selezionato 25 pazienti, trattati fra il 1997 e il 2006, ai quali era stato diagnosticato un carcinoma laringeo con stadiazione T3N0, che avevano rifiutato la laringectomia come possibile trattamento. Tutti i pazienti sono stati sottoposti ad una dose di 70 Gy di radioterapia (2Gy per frazione per 5 giorni alla settimana) in combinazione con 6 sessioni di ipertermia a cadenza settimanale. I pazienti sono stati inoltre sottoposti ad un trattamento chemioterapico settimanale con cis-platino. L'ipertemia è stata ottenuta mediante un generatore di microonde a 433 MHz con un sistema direzionale raffreddato ad acqua. La temperatura è stata monitorata a livello sottocutaneo in corrispondenza della porzione terminale del sistema direzionale per le microonde. La mediana del follow-up è stata di 60 mesi, nei quali 23 dei nostri 25 pazienti (92%) ha presentato una remissione completa. Nei due pazienti non responsivi alla termo-radioterapia si è proceduto all'esecuzione di una laringectomia totale, ed in corso di follow-up entrambi sono risultati essere vivi e liberi da malattia. Se valutata secondo i criteri dell'EORTC/RTOG la tossicità rilevata è stata leggera: 3 pazienti (12%) hanno presentato un grado III di tossicità cutanea acuta, otto (32%) un grado II e 14 (56%) un grado I. In due pazienti (8%) è stato registrato, a 6-8 mesi dalla termo-chemioterapia una tossicità laringea ritardata di grado III, con edema severo delle corde vocali. La temperatura minima registrata ha mostrato una correlazione sia con la risposta al trattamento (Spearman rho, p < 0,05) sia con la tossicità cutanea acuta e la funzione laringea. Nei casi in cui un paziente con un carcinoma T3N0 della laringe rifiuta la laringectomia, la termo-chemioterapia si propone come un alternativa efficace caratterizzata da effetti collaterali tollerabili quali la tossicità cutanea radioindotta o effetti indesiderati tardivi. La conferma dei nostri dati richiede tuttavia l'analisi di una casistica più ampia.
Introduction
Hyperthermia, the elevation of tumour temperature above 42°C, is an anticancer modality that can be used for the treatment of many types of cancer. Hyperthermia is usually applied in conjunction with other therapeutic modalities such as radiotherapy, as their mechanisms of action are complimentary 1. It has been observed that at high temperatures cell death is mainly due to protein denaturation which causes alteration in cell structures and changes in enzyme complexes that are required for DNA synthesis and repair 2. Radioresistant cancer cells that are hypoxic, acidotic and nutrient deprived, are sensitive to hyperthermia. In addition, hyperthermia and radiotherapy are effective in different phases of the cell cycle. Hyperthermia affects tumour blood flow and oxygenation resulting in enhancement of radiation response 3. Many trials have shown that the combined therapy is effective for the treatment of cancer and improves clinical response and local control of disease 4 5.
Advanced stage laryngeal carcinoma is associated with poor prognosis and greatly affects the patients' quality of life 6. The most common treatment modalities are either laryngectomy followed by chemoradiotherapy or radiotherapy alone 7. The incidence of laryngectomy post-radiotherapy due to local relapse for T3 laryngeal carcinoma remains high 7 8. Chemoradiotherapy offers improved clinical outcome in comparison to irradiation alone 9-11. There are patients, however, who deny the above therapeutic approach due to the poor quality of life caused by laryngectomy.
The purpose of our study was to test in a retrospective manner, whether the combination of hyperthermia and irradiation is an effective treatment modality for patients who suffer from T3N0 laryngeal carcinoma and refuse laryngectomy.
Materials and methods
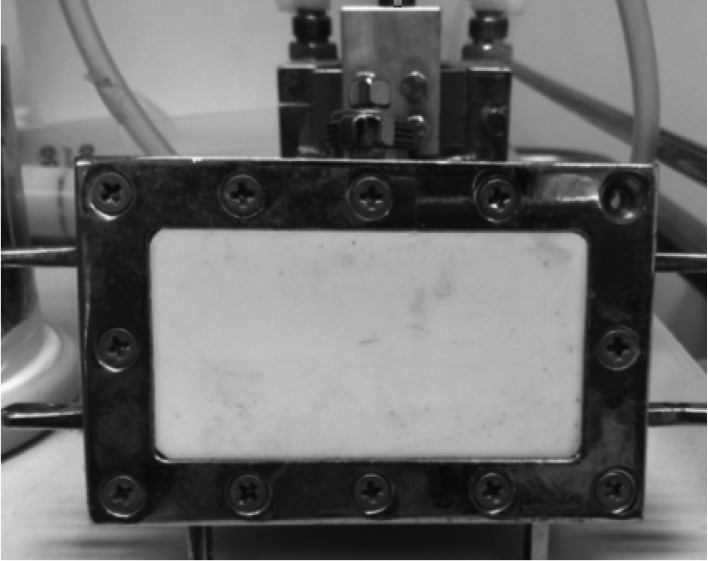
From 1997-2006, 25 patients diagnosed with T3N0 laryngeal carcinoma who denied laryngectomy were selected to participate in this retrospective study. The pre-treatment evaluation included blood test, clinical examination and CT scan of the cervix and thorax. The treatment of hyperthermia took place either in the Aretaieion University Hospital or at the Radiotherapy Department of YGEIA Hospital. The treatment planning used was either PLATO (Nucletron, The Netherlands) or HELAX-TMS (Nucletron B.V., Veenendaal, The Netherlands) depending on the radiotherapy department where irradiation was performed. The technique used in all cases was 3D conformal radiotherapy with CT-based image treatment planning. On all cases, the treatment delivery was performed with a 6MV LINAC SIEMENS MX. There were three phases of irradiation according to the technique of shrinking fields. In the first phase, together with the primary disease, the levels of IIA, IIB, III and IV were included up to 46 Gy, although no evidence for nodal disease was confirmed. However, this was done due to clinical (radiological) staging of the disease and not surgical (pathological) confirmation. Phase 2 included the laryngeal region with levels IIA and III (due to the close location with the larynx) cervical lymph nodes up to 56-58 Gy. The third phase included only the larynx up to 70 Gy. During phases 2 and 3, the spinal cord was excluded from the irradiation fields. An immobilization mask was used in all cases, while a conventional simulation was used before irradiation for confirmation of portals. In all patients, portal film was taken once per week throughout the entire radiotherapy schedule. Patients received a total dose of 70 Gy (2 Gy per fraction, 5 days per week) in combination with 6 weekly sessions of microwave hyperthermia. The hyperthermia session was performed weekly every Monday or Tuesday throughout the radiotherapy, at the left or the right side of the neck alternately by the weekly session. The hyperthermia device was operated as a 433 MHz microwave heating with water-loaded and water-cooled waveguides 12. The applicator used was a rectangular waveguide 6 × 7 cm with an effective field size of 5 × 6 cm (Fig. 1). The microwave device had an omitted power of 100 Watts RMS. The temperature was monitored with a thermocouple, while temperature measurement was made with 2 sec of power interruption. The temperature was monitored subcutaneously in the skin under the aperture of the waveguide. Thermal parameters such as Tmin and Tmax were always monitored. The hyperthermia session was prescribed as an one-hour heating with temperature range between 42-45°C. Radiation induced toxicity was evaluated with the EORTC/RTOG toxicity criteria 13.
Fig. 1.
Rectangular waveguide operating at 433 MHz used for local hyperthermia.
All patients received concurrent weekly cisplatin 40 mg/m2 as a radio-sensitizing agent for radiotherapy. All patients signed informed consent for the combined treatment according to the Declaration of Helsinki for Human Rights. All patients signed an informed consent that they agreed to have a combination of chemotherapy, radiotherapy and hyperthermia since they have denied surgical intervention. They were informed that the best practice for them would be laryngectomy, while the response to chemoradiotherapy and hyperthermia would not be the same as local excision. Moreover, they were informed about the potential toxicity of the combined treatment. Since there was no reimbursement for hyperthermia, the local board approved the inclusion of hyperthermia, while patients for this certain tumour location received 1-hour local thermotherapy without charge.
According to the study protocol, patients that were included should meet the following criteria:
Histologically proven glottic T3 squamous cell carcinoma.
Contrast – enhanced CT scan performed before treatment.
Confirmation of clinical staging of cervical lymph nodes, in terms of N0, with MRI study of the neck.
Normal electrocardiogram and normal chest–wall X– ray.
Age 18-80 years old and life expectancy > 2 months.
Karnofsky performance status (KPS) > 70% and World Health Organization Status (WHO) 0 - 1.
-
Laboratory values (performed one week before study):
Absolute neutrophil count > 3000/mm3;
Platelet count > 100,000/mm3;
Haemoglobin > 10 g/dl.
Urea and serum creatinine lower than upper limit of laboratory normal.
Total and direct bilirubin lower than upper limit of laboratory normal.
Serum glutamic – oxaloacetic transaminase, serum glutamic – pyruvic transaminase lower than upper limit of laboratory normal.
Alkaline phosphatase lower than upper limit of laboratory normal.
Patient denial for laryngectomy.
The exclusion criteria for the study were:
Age > 80 years old.
Patients with pacemaker.
Patients with implanted electronic devices.
Patients with implanted stents.
Patients with fever before treatment.
The primary endpoints were response to treatment according to RECIST criteria 14 together with loco-regional control rate and overall survival. The secondary endpoint was treatment-related toxicity. All patients underwent CT and/or MRI study together with direct laryngoscopy every 3 months the first year and every six months thereafter. According to the local protocol in our department for follow-up in head and neck patients, the intention was to follow-up patients for 5 years post-treatment.
Statistical analysis
The correlation between thermal parameters and toxicity grading (acute and late) as well as response rate was performed with the Spearman rho non-parametric test. The Kaplan-Meier method was used for survival analysis. Comparison of KPS values before and after treatment was done with the Wilcoxon non-parametric test. The significance level in all cases was taken at the level of 0.05. All analyses were performed with SPSS ver. 10 (IL, USA).
Results
In our study, 25 patients with laryngeal carcinoma who received local hyperthermia combined with chemo-radiotherapy were included in this retrospective study. According to CT images before treatment, the mean tumour volume was 3.26 (± 0.19) cm3, with a range of 2.9-3.5 cm3. The median follow-up was 60 months (range 60-72 months), while 23 of 25 patients (92%) presented complete response to treatment. Concerning the two failures, one presented stable disease at three months post-treatment, while the other had progressive disease at 6 months post-treatment. The two patients that did not respond to thermoradiotherapy underwent total laryngectomy with clear margins and lymph node dissection of levels II and III, with pathological confirmation of N0 disease. During follow-up these patients remained alive and free of disease. Toxicity was mild: three patients (12%) presented grade III, eight (32%) presented grade II and 14 (56%) presented grade I acute skin toxicity. There was an increased acute skin toxicity (mainly erythema) in the area of hyperthermia treatment at the footprint of the antenna. Moderate late toxicity consisted of vocal cord malfunction due to severe oedema (grade III), and was noted in two patients (8%) at 6-8 months post-thermoradiotherapy. The loco-regional control rate at 5 years is shown in Figure 2. Acute and late skin toxicity together with the late laryngeal toxicity related to laryngeal function is reported in Table I.
Fig. 2.
Loco-regional control rate for a median follow-up of five years.
Table I.
Acute/late skin toxicity and late laryngeal toxicity according to EORTC/RTOG criteria.
| Grade I | Grade II | Grade III | |
|---|---|---|---|
| Acute skin toxicity | |||
| Follicular, faint or dull erythema / epilation / dry desquamation / decreased sweating | Tender or bright erythema, patchy moist desquamation / moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | |
| 14/25 (56%) | 8/25 (32%) | 3/25 (12%) | |
| Late skin toxicity | |||
| Slight atrophy; pigmentation change | Patch atrophy; moderate telangiectasia | Marked atrophy; gross telangiectasia | |
| 5/25 (20%) | 2/25 (8%) | - | |
| Late laryngeal toxicity | |||
| Hoarseness; slight arytenoid oedema | Moderate arytenoid edema; chondritis | Severe oedema; severe chondritis | |
| 19/25 (76%) | 4/25 (16%) | 2/25 (8%) |
During the hyperthermia session, thermal parameters such as Tmin and Tmax were monitored for all patients. From our statistical analysis, it was found that Tmax correlated with Tmin (Spearman rho = 0.69, p < 0.01), acute skin toxicity (Spearman rho = 0.78, p < 0.01) and late reactions such as laryngeal function (Spearman rho = -0.45, p < 0.029). A correlation was found between Tmin and response to treatment (Spearman rho = -0.47, p < 0.017) as well as acute toxicity (Spearman rho = 0.54, p < 0.005) and laryngeal function (Spearman rho = -0.48, p = 0.021). All these correlations were statistically significant. Finally, statistically significant correlation was noted between laryngeal function and response as well as acute toxicity (Spearman rho = 0.74, p < 0.01 and Spearman rho = -0.52, p = 0.012 respectively). The results of the statistical analysis are presented in Table II. No haematological toxicity was seen in any case. The mean Karnofsky performance status score before and after combined treatment was 88.4% (± 8.9) and 88.2% (± 9.8), respectively (p = 0.805, Wilcoxon test).
Table II.
Spearman rho correlation between thermal parameters, response rate, EORTC/RTOG acute skin toxicity and laryngeal function.
| Tmax | Response (yes/no) | EORTC/RTOG (acute) | Laryngeal function (EORTC/RTOG late) |
||
|---|---|---|---|---|---|
| Tmin | rho p |
0.69 < 0.01 |
-0.47 0.017 |
0.54 0.005 |
-0.48 0.021 |
| Tmax | rho p |
-0.31 0.136 |
0.78 < 0.01 |
-0.45 0.029 |
|
| Response (yes/no) | rho p |
-0.25 0.223 |
0.74 < 0.01 |
||
| EORTC/RTOG (acute) | rho p |
-0.52 0.012 |
Discussion
The treatment of choice for laryngeal carcinoma consists of surgery in addition with chemoradiotherapy or radiotherapy alone 7 15. This treatment often causes toxicity and is related with limited local control and survival 6 15. Studies have shown that total laryngectomy has a survival advantage over chemoradiation or radiation alone. Chen 10 demonstrated that considering one-year survival, the hazard ratio (HR) is 1.0 for total laryngectomy, while for chemoradiotherapy is 1.35 and 1.51 for radiotherapy alone. Concerning 4-year survival, these ratios were 1.0, 1.24 and 1.39, respectively. Corry 7 showed that for patients treated with chemoradiotherapy without surgical treatment, overall survival was disappointing. Three-year overall survival and 5-year overall survival were 67% and 45%, respectively. The rates for 3-year local disease control and 5-year local disease control were 83% and 77%, respectively. In another study, patients treated with either induction chemotherapy and hyperfractionated radiation therapy or chemoradiation overall survival was 66%; an anatomically intact larynx was retained in 79% of patients, but 30% never resumed their pretreatment organ function 8. In addition, many trials demonstrate that chemoradiotherapy leads to better therapeutic results than radiotherapy alone 9 11 16-18.
Hyperthermia seems very promising in combination with radiotherapy, showing an enhancement in response to irradiation 19. Several studies have shown the potential radio-sensitization both in animals and in humans 20 21. In our study, we investigated if the combination of local hyperthermia with chemoradiotherapy in patients who denied laryngectomy is an effective treatment for T3N0 laryngeal cancer. We also assessed the acute and late toxicity of this therapeutic modality. Our results showed that for T3N0 the complete response rate was up to 92% after combined thermo-chemoradiotherapy. Only two patients did not respond to treatment and underwent total laryngectomy. Hinohira 22 showed that for patients suffering from laryngeal carcinoma and treated with hyperthermia and chemoradiation, the response rate (complete and partial response) was 100%. Other studies testing the combination of irradiation and hyperthermia in patients suffering from head and neck cancer showed that the complete response rates ranged from 76-78.6% 23-26. The trials of Engin 27 and Gabriele 28 showed complete response rates of 58% and 40%, respectively. In the trial of Serin, the local complete response rate of patients treated with hyperthermia and chemoradiotherapy was 82% 29. Chang 30 treated patients with head and neck cancer (stage IV) with a combination of hyperthermia and chemotherapy with a response rate (complete and partial response) of 37.5%. The median follow-up of our patients was 60 months, and a significant 5-year survival rate was observed. In comparison with the above mentioned studies, treatment outcome in our study appears to be high (response rate up to 92%), whereas the rates for 5-year survival referred to in the current literature concerning thermoradiotherapy are 24%, 50-55% and 68.2% 23 24 31.
The acute toxicity observed by combined treatment was mild; vocal cord malfunction was noted as late toxicity at 6-8 months post thermoradiotherapy in only two patients. It is well established in many trials that hyperthermia in addition to radiation therapy is well tolerated and is not associated with severe side effects 23 27 28.
In studies that tested the correlation between thermal parameters of hyperthermia and clinical outcome of patients with head and neck cancer, there was an impact of thermoradiotherapy on both therapeutic outcome 28 and morbidity 27, although this correlation did not reach statistical significance. From our statistical analysis, it was found that Tmax correlated with Tmin (Spearman rho = 0.69, p < 0.01) and acute (Spearman rho = 0.78, p < 0.01) and late reaction-laryngeal function (Spearman rho = -0.45, p < 0.029). A correlation was also found between Tmin with response (Spearman rho = -0.47, p < 0.017), acute toxicity (Spearman rho = 0.54, p < 0.005) and laryngeal function (Spearman rho = -0.48, p = 0.021). All these correlations were statistically significant. In a previous study 32, we reported on the impact of Tmin with response of superficial tumours. In addition, the reason for the correlation of Tmin with high radiation induced morbidity might be the elevated temperatures affecting both skin tolerability and glottic cord functionality, in terms of the radiosensitivity that the thermotherapy produces in normal tissues. Finally, statistically significant correlation was noted between laryngeal function as well as response and acute toxicity (Spearman rho = 0.74, p < 0.01 and Spearman rho = -0.52, p = 0.012, respectively). The reason for this is obviously the highly deposited microwave power in both the glottic tumour and the normal surrounding tissues, since hyperthermia has the disadvantage of the lack of focusing only in cancerous tissue. The effective field size of our hyperthermia applicator is 5 × 6 cm, by including both anatomically normal structures and the glottic tumour in the hyperthermia field.
Hyperthermia seems to play an important role in the cure of head and neck cancer, since it was observed that when hyperthermia is added to radiotherapy the response rates are significantly higher that those of radiation therapy alone. Valdagni, reported the results of a randomized study on 44 metastatic squamous cell cervical lymphnodes comparing radical irradiation vs. radical irradiation plus twice a week local microwave hyperthermia 33. They showed that the complete response rate for patients treated with irradiation alone was 37% and increased to 82% with the addition of hyperthermia. Huilgol 26, in a randomized trial with 56 patients, found a statistically significant difference in complete response: 42.4% for irradiation alone and 78.6% for thermoradiotherapy. Hoshima 31, comparing thermochemoradiotherapy in 18 patients with 25 recurrent lesions vs. chemoradiotherapy in 22 patients with 27 recurrent lesions, reported similar results with combined thermo-chemotherapy with a total response rate up to 92.0%, while there was a significant difference in complete response between the two groups. In the same study, the 5-year cumulative local control was 68.2% vs. 22.2% in favour of hyperthermia.
Several studies have been published on radical irradiation ± chemotherapy instead of laryngectomy for T3N0 disease. Hinerman 34 studied 68 patients who received radiotherapy without chemotherapy and reported a local control rate up to 78% and overall survival up to 52%. Bergqvist 35 retrospectively reported a 5-year survival rate up to 72% for patients who underwent radiotherapy. Jackson 36, in 70 patients with T3 disease, reported a 5-year recurrence free rate of 65% with radiotherapy alone. It should not be underestimated that the high loco-regional response rate in our study with complete response was up to 92%. There are three possible reasons for this result. In our opinion, the first is the addition of local hyperthermia to the laryngeal area. The second is the fact that all of our patients had a tumour volume less than 3.5 cm3. Indeed, Lee 37 as well as Pameijer 38 reported excellent results with larynx preservation up to 85-92% when tumour volume was less than 3.5 cm3, such as in our case. The third is the prophylactic irradiation of clinical N0 lymph nodes, even without surgical dissection and consequent pathological staging. In fact, Yu 39, in 83 patients with T3-4 laryngeal tumours, reported a neck recurrence rate of clinical N0 patients of 14.3%. It should not be underestimated that the frequency used for local hyperthermia was 433 MHz instead of 915 MHz, which is commercially used for superficial heating. The approximate penetration depth for 433 MHz and 915 MHz is 3.5 cm and 2 cm respectively, by deeper heating with our applicator.
Other than the small number of patients and the retrospective inclusion of the patients, the main weakness of the present study is the lack of objective temperature measurements in the tumour, since monitoring with the thermocouple was performed on the skin surface. However, it seems difficult to put a thermocouple in the larynx of the patient without general anaesthesia. Although it was not available in our clinical study, non-invasive thermometry with magnetic resonance using proton resonance frequency shift 40 would be a potential solution to this problem.
Conclusions
In the present study, we report our experience with combined hyperthermia and chemoradiotherapy for T3N0 squamous cell carcinoma of the glottis. This retrospective study covers a long period of time, due to the low number of patients who met the inclusion criteria. Indeed, the incidence of patients with T3N0 laryngeal carcinoma who denied laryngectomy is low, although all consecutive patients were referred in our department from several centres in Athens.
The quality of life for patients after laryngectomy and radiotherapy is definitely worse than those with chemoradiotherapy 41. However, the quality of life is sometimes worse in patients who underwent combined and multimodality therapy rather than monotherapy with surgery or radiotherapy alone, while in some cases patients considered the laryngectomy as a liberation 42. However, in our case, patients simply avoided laryngectomy as their first line of treatment. The response rate of 92% reported here is one of the highest in the current literature, especially for T3N0 glottic carcinoma, where the commonly-reported response rate is 60-70% 7-11 16-18 36 43. Although no quality of life measurements were performed in our study, the KPS score did not change significantly before and 3 months post-combined treatment. When the patient with T3N0 laryngeal carcinoma denies laryngectomy, an alternative treatment can be combined thermo-chemoradiotherapy, which seems effective and generally tolerable although it is associated with radiation induced skin toxicity and late side effects.
Our results seem encouraging, and more patients are needed to confirm the effectiveness and toxicity of this combined treatment for cure of laryngeal carcinoma. However, due to the retrospective design of the present study, it should be mentioned that no definite conclusions can be reached, and that the final results are likely to be related to hyperthermia only, since a tri-modality treatment was applied to all cases. Thus, a randomized study with chemoradiotherapy vs. thermo-chemoradiotherapy is needed to confirm confirmation these results. Lastly, the danger for bias in a retrospective study is always present: in our case, all patients had a tumour volume less than 3.5 cm3 which is a favourable factor.
References
- 1.Kouloulias VE. Hyperthermia in solid tumors. Aktinotechnologia. 2003;12:38–45. [Google Scholar]
- 2.Zee J. Heating the patient: a promising approach? Ann Oncol. 2002;13:1173–1184. doi: 10.1093/annonc/mdf280. [DOI] [PubMed] [Google Scholar]
- 3.Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005;23:3079–3085. doi: 10.1200/JCO.2005.05.520. [DOI] [PubMed] [Google Scholar]
- 4.Zee J, Vujaskovic Z, Kondo M, et al. The Kadota Fund International Forum 2004-Clinical Group consensus. Int J Hyperthermia. 2008;24:111–122. doi: 10.1080/02656730801895058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engin K, Leeper DB, Tupchong L, et al. Thermoradiotherapy in the management of superficial malignant tumors. Clin Cancer Res. 1995;1:139–145. [PubMed] [Google Scholar]
- 6.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corry J, Rischin D, Cotton S, et al. Larynx preservation with primary non-surgical treatment for loco-regionally advanced larynx cancer. J Med Imaging Radiat Oncol. 2011;55:229–235. doi: 10.1111/j.1754-9485.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 8.Guadagnolo BA, Haddad RI, Posner MR, et al. Organ preservation and treatment toxicity with induction chemotherapy followed by radiation therapy or chemoradiation for advanced laryngeal cancer. Am J Clin Oncol. 2005;28:371–378. doi: 10.1097/01.coc.0000162423.13431.8d. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116:1–13. doi: 10.1097/01.mlg.0000236095.97947.26. [DOI] [PubMed] [Google Scholar]
- 10.Chen AY, Fedewa S, Pavluck A, et al. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116:4744–4752. doi: 10.1002/cncr.25364. [DOI] [PubMed] [Google Scholar]
- 11.Gourin CG, Conger BT, Sheils WC, et al. The effect of treatment on survival in patients with advanced laryngeal carcinoma. Laryngoscope. 2009;119:1312–1317. doi: 10.1002/lary.20477. [DOI] [PubMed] [Google Scholar]
- 12.Uzunoglu NK, Angelikas EA, Cosmidis PA. A 432-MHz Local hyperthermia system using an indirectly cooled, waterloaded waveguide applicator. IEEE Transactions on Microwave Theory and Techniques. 1987;35:106–111. [Google Scholar]
- 13.Cox JD, Stenz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer. Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumours. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre J-L. Larynx preservation. Curr Opin Oncol. 2012;24:218–222. doi: 10.1097/CCO.0b013e3283523c95. [DOI] [PubMed] [Google Scholar]
- 16.Hartl DM, Ferlito A, Brasnu DF, et al. Evidence-based review of treatment options for patients with glottic cancer. Head Neck. 2011;33:1638–1648. doi: 10.1002/hed.21528. [DOI] [PubMed] [Google Scholar]
- 17.Wolf GT, Hong WK, Gross Fisher S. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 18.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 19.Triantopoulou S, Efstathopoulos E, Platoni K, et al. Radiotherapy in conjunction with superficial and intracavitary hyperthermia for the treatment of solid tumors: survival and thermal parameters. Clin Transl Oncol. 2013;15:95–105. doi: 10.1007/s12094-012-0947-3. [DOI] [PubMed] [Google Scholar]
- 20.Sawas-Dimopoulou C, Papavasilion C, Iordanou I, et al. Comparative evaluation of combined irradiation and hyperthermia versus irradiation alone. Eur Radiol. 1994;4:167–171. [Google Scholar]
- 21.Zee J, Vujaskovic Z, Kondo M, et al. The Kadota Fund International Forum 2004 – clinical group consensus. Int J Hyperthermia. 2008;24:111–122. doi: 10.1080/02656730801895058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinohira Y, Yumoto E, Hyodo M, et al. Thermoradiotherapy combined with daily administration of low dose cisplatin for locally advanced laryngeal carcinoma. Gan To Kagaku Ryoho. 1997;24:1961–1965. [PubMed] [Google Scholar]
- 23.Amichetti M, Romano M, Busana L, et al. Hyperfractionated radiation in combination with local hyperthermia in the treatment of advanced squamous cell carcinoma of the head and neck: a phase I-II study. Radiother Oncol. 1997;45:155–158. doi: 10.1016/s0167-8140(97)00134-5. [DOI] [PubMed] [Google Scholar]
- 24.Amichetti M, Zurlo A, Cristoforetti L, et al. Prognostic significance of cervical lymph nodes density evaluated by contrasted computer tomography in head and neck squamous cell carcinoma treated with hyperthermia and radiotherapy. Int J Hyperthermia. 2000;16:539–547. doi: 10.1080/02656730050199377. [DOI] [PubMed] [Google Scholar]
- 25.Huilgol NG, Gupta S, Dixit R. Chemoradiation with hyperthermia in the treatment of head and neck cancer. Int J Hyperthermia. 2010;26:21–25. doi: 10.3109/02656730903418283. [DOI] [PubMed] [Google Scholar]
- 26.Huilgol NG, Gupta S, Sridhar CR. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: a report of randomized trial. J Cancer Res Ther. 2010;6:492–496. doi: 10.4103/0973-1482.77101. [DOI] [PubMed] [Google Scholar]
- 27.Engin K, Tupchong L, Waterman FM, et al. Thermoradiotherapy for superficial tumour deposits in the head and neck. Int J Hyperthermia. 1994;10:153–164. doi: 10.3109/02656739409009340. [DOI] [PubMed] [Google Scholar]
- 28.Gabriele P, Ferrara T, Baiotto B, et al. Radio hyperthermia for re-treatment of superficial tumours. Int J Hyperthermia. 2009;25:189–198. doi: 10.1080/02656730802669593. [DOI] [PubMed] [Google Scholar]
- 29.Serin M, Erkal HS, Cakmak A. Radiation therapy, cisplatin and hyperthermia in combination in management of patients with carcinomas of the head and neck with N2 or N3 metastatic cervical lymph nodes. Radiother Oncol. 1999;50:103–106. doi: 10.1016/s0167-8140(98)00098-x. [DOI] [PubMed] [Google Scholar]
- 30.Chang P, Sapozink MD, Grunberg SM, et al. Unresectable primary and recurrent head and neck tumors: effect of hyperthermia and carboplatin--preliminary experience. Radiology. 2000;214:688–692. doi: 10.1148/radiology.214.3.r00mr51688. [DOI] [PubMed] [Google Scholar]
- 31.Hoshina H, Takagi R, Tsurumaki H, et al. Clinical result of thermochemoradiotherapy for advanced head and neck cancer. Gan To Kagaku Ryoho. 2001;28:331–336. [PubMed] [Google Scholar]
- 32.Kouloulias V, Dardoufas C, Kouvaris J, et al. Combined radiotherapy and hyperthermia in the treatment of superficial carcinomas. The experience of the University of Athens Medical School. J BUON. 2001;6:245–253. [Google Scholar]
- 33.Valdagni R, Amichetti M, Pani G. Radical radiation alone versus radical radiation plus microwave hyperthermia for N3 (TNM-UICC) neck nodes: a prospective randomized clinical trial. Int J Radiat Oncol Biol Phys. 1988;15:13–24. doi: 10.1016/0360-3016(88)90341-0. [DOI] [PubMed] [Google Scholar]
- 34.Hinerman RW, Mendenhall WM, Morris CG, et al. T3 and T4 true vocal cord squamous carcinomas treated with external beam irradiation: a single institution's 35-year experience. Am J Clin Oncol. 2007;30:181–185. doi: 10.1097/01.coc.0000251368.57302.cc. [DOI] [PubMed] [Google Scholar]
- 35.Bergqvist M, Brodin O, Pouzon A, et al. Radiation treatment of T1-T4 squamous cell carcinoma of the larynx: a retrospective analysis and long-term follow-up of 135 patients. Anticancer Res. 2002;22:1239–1242. [PubMed] [Google Scholar]
- 36.Jackson SM, Hay JH, Flores AD. Local control of T3N0 glottic carcinoma by 60 Gy given over five weeks in 2.4 Gy daily fractions. One more point on the biological effective dose (BED) curve. Radiother Oncol. 2001;59:219–220. doi: 10.1016/s0167-8140(01)00286-9. [DOI] [PubMed] [Google Scholar]
- 37.Lee WR, Mancuso AA, Saleh EM, et al. Can pretreatment computed tomography findings predict local control in T3 squamous cell carcinoma of the glottic larynx treated with radiotherapy alone? Int J Radiat Oncol Biol Phys. 1993;25:683–687. doi: 10.1016/0360-3016(93)90016-o. [DOI] [PubMed] [Google Scholar]
- 38.Mendenhall WM, Parsons JT, Mancuso AA, et al. Definitive radiotherapy for T3 squamous cell carcinoma of the glottic larynx. J Clin Oncol. 1997;15:2394–2402. doi: 10.1200/JCO.1997.15.6.2394. [DOI] [PubMed] [Google Scholar]
- 39.Yu WB, Zeng ZY, Chen FJ, et al. Correlation of cervical lymphatic metastasis to prognosis of T3-T4 glottic cancer. Ai Zheng. 2006;25:1271–1274. [PubMed] [Google Scholar]
- 40.Ludemann L, Wlodarczyk W, Nadobny J, et al. Non-invasive magnetic resonance thermography during regional hyperthermia. Int J Hyperthermia. 2010;26:273–282. doi: 10.3109/02656731003596242. [DOI] [PubMed] [Google Scholar]
- 41.Terrell JE, Fisher SG, Wolf GT. Long-term quality of life after treatment of laryngeal cancer. The Veterans Affairs Laryngeal Cancer Study Group. Arch Otolaryngol Head Neck Surg. 1998;124:964–971. doi: 10.1001/archotol.124.9.964. [DOI] [PubMed] [Google Scholar]
- 42.Succo G, Bramardi F, Airoldi M, et al. Quality of life after treatment in patients with laryngeal carcinoma. Acta Otorhinolaryngol Ital. 1997;17:32–44. [PubMed] [Google Scholar]
- 43.Bourhis J, Lefebvre JL, Temam S, et al. Larynx preservation: nonsurgical approaches. Cancer Radiother. 2004;8:24–28. [PubMed] [Google Scholar]