Fig. 2.
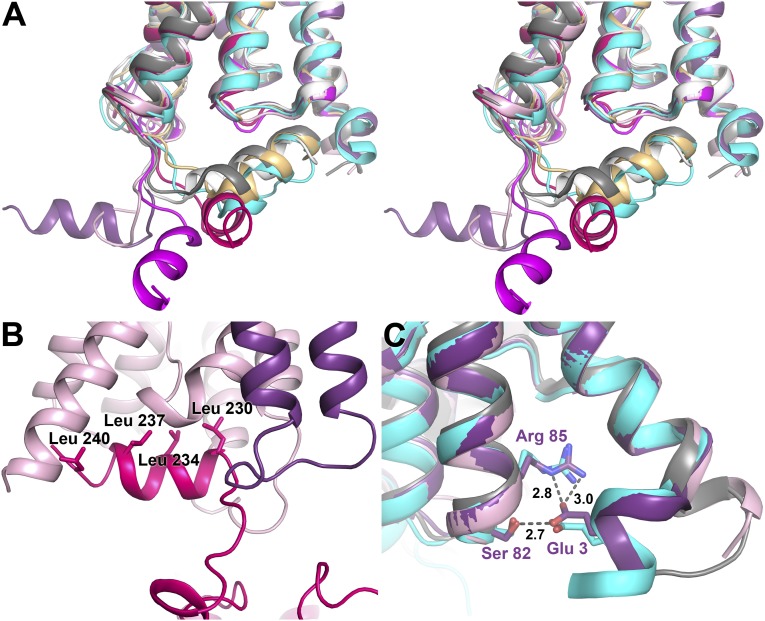
Structure of the C and N termini. (A) Stereo image of the four AQP2 protomers (colored as in Fig. 1) overlaid on OaAQP0 [white; Protein Data Bank (PDB) ID code 2B60], BtAQP0 (light orange; PDB ID code 1YMG), BtAQP1 (gray; PDB ID code 1J4N), and HsAQP5 (cyan; PDB ID code 3D9S). The C termini of all four AQP2 protomers occupy different positions, none of which overlay with any of the previous AQP structures. (B) The crystal contact site between the C-terminal helix of monomer C (pink) and a symmetry-related protomer D (light pink). Leucines lining up on one side of the helix are labeled. (C) Overlay of the N termini of protomer A and D with HsAQP5 (cyan) and BtAQP1 (gray). For protomer A (purple), Glu3 interacts with Ser82 and Arg85, similar to the structural arrangement seen in AQP5. In contrast, protomer D (light pink) resembles BtAQP1 with TM helix 1 extending a full turn into the cytoplasm.