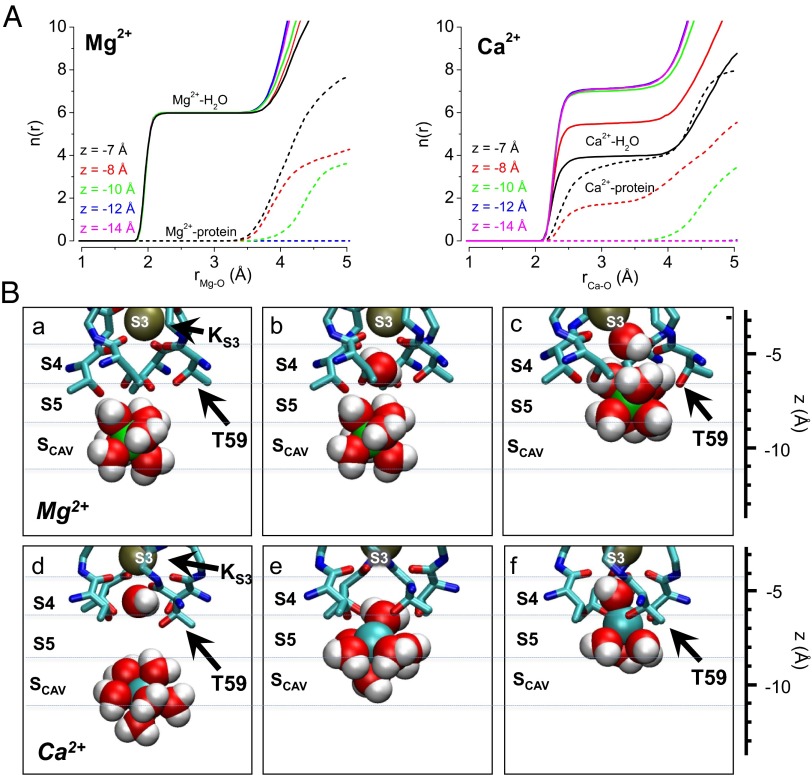
Fig. 6.
Structural basis for Ca2+ access and binding at the S5 site. (A) Number of oxygen atoms within radius r [n(r)] of (Left) Mg2+ and (Right) Ca2+; n(r) is shown separately for water and protein oxygen (solid and dashed lines, respectively), and each curve represents the relative position of the ion along the central axis of the MthK pore (z). These data show that Mg2+ remains hydrated with up to six water molecules at these positions in the MthK pore (coordination distance between 2 and 3.5 Å), whereas Ca2+ can exchange three to four water molecules for protein oxygen as it approaches the S5 site (z between −7 and −8 Å). (B) Representative snapshots from MthK pore simulations with (a–c) Mg2+ (green spheres) and (d–f) Ca2+ (cyan spheres) with nearby water molecules (red spheres, oxygen; white spheres, hydrogen) and selectivity filter atoms (shown as sticks with side chain of Thr-59 indicated by black arrows). These snapshots illustrate that the energy barrier impeding Mg2+ access to S5 arises primarily from its strong interaction with hydration shell water molecules, which leads to overall steric hindrance by selectivity filter atoms (c), whereas Ca2+ exchanges its hydration shell water molecules in favor of the hydroxyl side chains of T59 (f).