Figure 1.
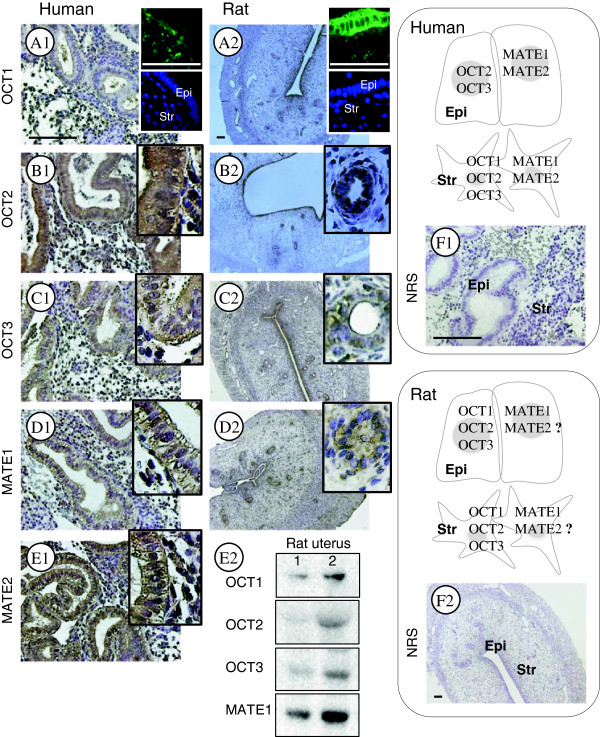
Comparison of endogenous OCT1, OCT2, OCT3, MATE1, and MATE2 localization in human endometria and rat uterine tissues. Human endometrial biopsies (n = 4) and rat uteri (n = 6) were fixed in formalin and embedded in paraffin. Rabbit anti-OCT1 (AV41516, 1:100 dilution for human and rat), rabbit anti-OCT2 (HPA008567, 1:100 for human, 1:200 for rat), and rabbit anti-MATE1 (HPA021987, 1:100 for human, 1:200 for rat) were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Rabbit anti-OCT3 (ab183071, 1:25 for human, 1:100 for rat) and rabbit anti-MATE2 (ab106117, 1:100 for human) were obtained from Abcam (Cambridge, UK). The localization of OCT1–3 and MATE1 and 2 was observed with a peroxidase-antiperoxidase detection method using a single 3,3'-diaminobenzidine (DAB) as the chromogen. Non-specific binding was blocked with Background Sniper (Biocare Medical, CA, USA). Representative micrographs show strong OCT1 immunoreactivity in stromal cells but not in epithelial cells in human endometria (A1). In contrast, OCT1 immunoreactivity is detected in both epithelial and stromal cells in the rat uterus, and there is greater OCT1 immunoreactivity in the epithelial cells (A2). Representative micrographs show that immunoreactivity of OCT2, OCT3, MATE1, and MATE2 is detected in the epithelial and stromal cells in human endometria (B1–E1) and the rat uterus (B2–D2). An antibody against rat MATE2 is not commercially available so this was not tested. Immunofluorescent images of OCT1 are shown in the upper right corner of A1 and A2 and were used to confirm the immunohistochemical analysis. Sections that were exposed to rabbit normal serum were used as negative controls (F1 and F2). Hematoxylin was used to identify the cell nuclei. Epi, epithelial cells; Str, stromal cells; NRS, normal rabbit serum. Scale bar, 100 μm. Different rat uterine tissue lysates were directly immunoblotted with antibodies against OCT1, OCT2, OCT3, or MATE1 as indicated in E2.
