Figure 2.
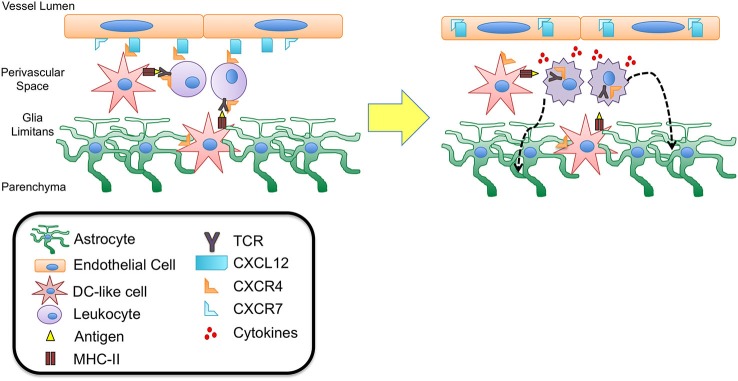
CXCL12 fate determination of perivascular T cells. DC-like cells have been identified in the perivascular space as well as in the juxtavascular parenchyma abutting astrocytic end feet and extending processes into the basement membrane of the glia limitans. These cells express CD11c but lack MHC II expression suggesting they may be quiescent DCs capable of recognizing antigen during routine immune surveillance. It is possible that upon antigen uptake these cells are activated to up-regulate MHC II expression as well chemokine receptors such as CXCR4. Interaction with CXCL12 expressed at the abluminal side of the BBB endothelium could thus function to retain these cells as well as circulating lymphocytes within the perivascular spaces, facilitating antigen presentation and recognition. Upon binding to antigen presented in MHC II complexes, the T cell antigen receptor (TCR) expressed on T cells heterodimerizes with CXCR4, initiating intracellular signaling cascades that lead to T cell activation and migration. Recent studies have demonstrated that TCR-activated β-arrestin signaling down-regulates T cell surface CXCR4 expression, perhaps removing an important retention cue within the perivascular niche, thereby promoting entry of T cells into deeper parenchymal tissue. This process may also be promoted by the release of cytokines due to TCR binding and T cell activation. Inflammatory cytokines have been shown to up-regulate levels of CXCL12 as well as the scavenger chemokine receptor CXCR7. CXCR7 is capable of binding to and internalizing CXCL12, leading to loss of polarity at the BBB. Relocation of CXCL12 from abluminal to luminal endothelial surfaces at the BBB has been shown to lead to extensive leukocyte infiltration and neuroinflammation in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS).
