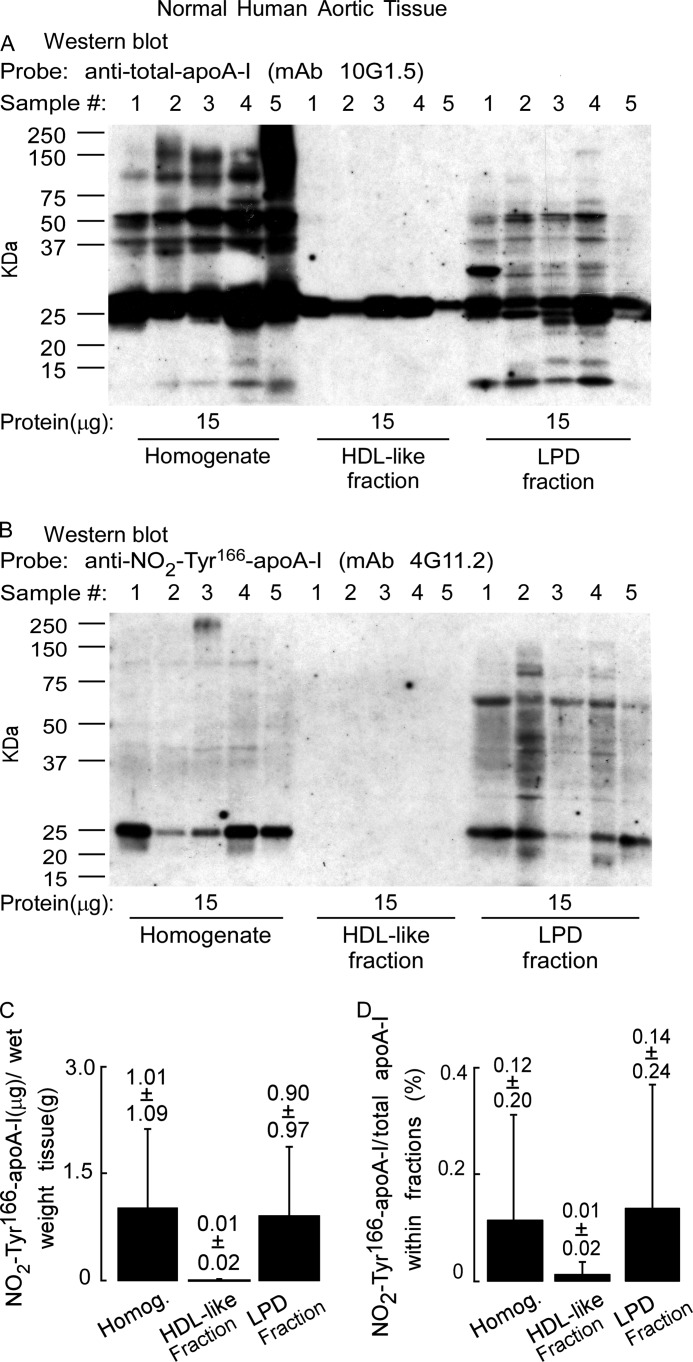
FIGURE 8.
ApoA-I nitrated on tyrosine 166 obtained from the normal aortic tissue is not on an HDL-like particle. A, aortic tissue that appears histologically normal was used to prepare a tissue homogenate as described under “Experimental Procedures.” The homogenate was fractionated on sequential D2O/sucrose buoyant density gradients by ultracentrifugation, the indicated amounts of protein were separated by gradient (5–15%) reducing SDS-PAGE, and proteins were transferred to membranes. A, Western blot of normal artery wall proteins (15 μg) from homogenate and HDL-like and LPD fractions probed with anti-total apoA-I antibody mAb 10G1.5 to detect total apoA-I in homogenate and HDL-like and LPD fractions. B, duplicate Western blot as in A probed with anti-NO2-Tyr166-apoA-I mAb 4G11.25 to show anti-NO2-Tyr166-apoA-I only in the starting material and in the LPD fraction. C, recovery of NO2-Tyr166-apoA-I (μg) per gram of normal aortic tissue (wet weight) that was found to be present in the homogenate (Homog.) and in the HDL-like and LPD fractions was determined from quantitative Western blot analysis of apoA-I-immunoreactive bands and total apoA-I content as described under “Experimental Procedures” and is qualitatively presented in Fig. 9. D, percentage of NO2-Tyr166-apoA-I to total apoA-I from normal aortic tissue present in the starting homogenate and HDL-like and LPD fractions was calculated from quantitative Western blot analysis of apoA-I-immunoreactive bands as calculated in C. Values were determined from n = 5 samples; error bars represent ±S.D.