FIGURE 8.
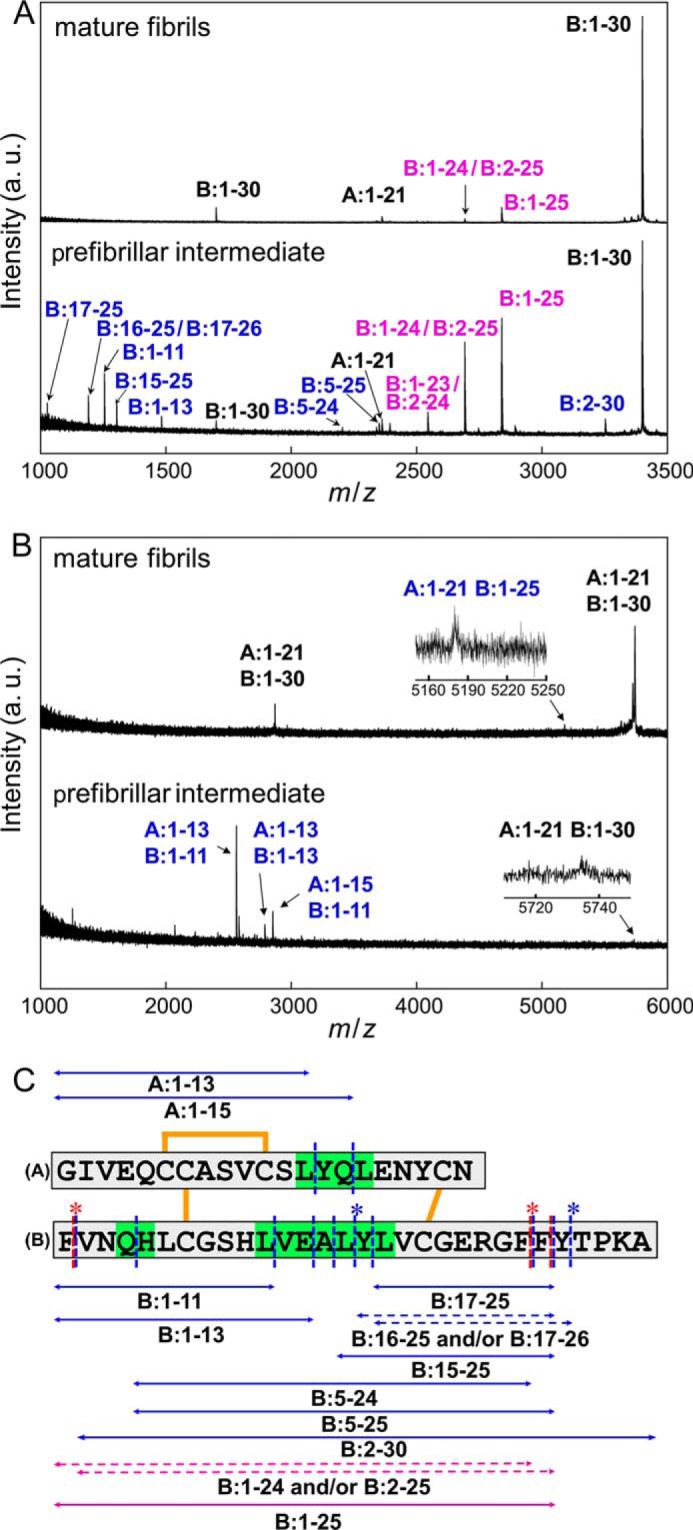
Identification of amino acid regions responsible for manifesting the propagation ability and cytotoxicity. A and B, MALDI-TOF mass spectra of peptide fragments of prefibrillar intermediate and mature fibrils. In this study, peptides were identified under two conditions: with (A) and without (B) the reduction of disulfide bonds inside the insulin molecule. The spectra of mature fibrils and prefibrillar intermediate species are shown on the upper and lower sides of each panel, respectively, and blue and magenta labels on the assigned peaks represent peptide fragments found only in the prefibrillar intermediate and in both the prefibrillar intermediate and mature fibrils, respectively. Black labels represent peaks derived from an uncleavaged fraction of insulin molecules. Also see Tables 1 and 2 for numerical data on the peaks detected. C, summary of the limited proteolysis of the intermediate and mature fibrils insulin by pepsin. The peptides identified by MALDI-TOF mass spectrometry are shown by double-headed arrows alongside the primary sequences of the A-chain and B-chain. The blue and magenta arrows represent peptide fragments found only in intermediate fibrils and those found in both intermediate and mature fibrils, respectively. Cleavage sites are summarized on the primary sequence of insulin, in which blue and red dashed lines represent the sites cleaved in the intermediate and mature fibrils, respectively. Regarding peptide fragments in which two possible identifications were assigned on the basis of the mass values, both of these possibilities were represented by dashed double-headed arrows with asterisks at the corresponding cleavage sites on the primary sequence. The regions sensitive to proteolytic digestion only in the intermediate fibrils are colored green.
