FIGURE 1.
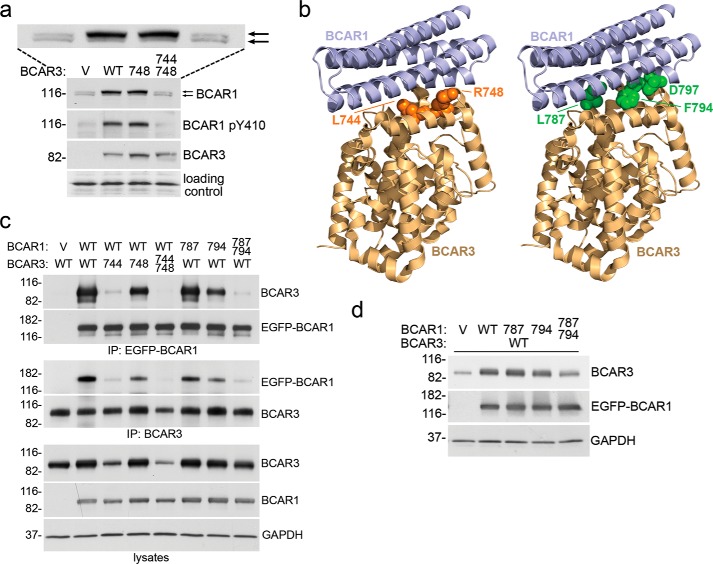
Structure-guided mutation of multiple residues in the binding surfaces of BCAR3 or BCAR1 disrupts their interaction and abrogates crucial signaling events. a, stable lentiviral transduction of BCAR3 wild-type (WT) or the L748E single mutant in MCF7 breast cancer cells similarly increase BCAR1 levels, BCAR1 substrate domain tyrosine phosphorylation (detected with an antibody to phospho-Tyr-410), and the proportion of the hyperphosphorylated BCAR1 upper band (top arrow). An enlargement of the BCAR1 blot is also included to more clearly show the upper and lower BCAR1 bands, which are marked by the two arrows. In contrast, the L744E/R748E double mutant has lost these activities. V, empty lentiviral vector control. Cell lysates were probed by immunoblotting with the indicated antibodies. A band recognized non-specifically by the BCAR1 antibody demonstrates equal loading of the lanes (loading control). b, BCAR1-BCAR3 complex modeled by overlaying the crystal structure of the BCAR3 C-terminal domain (PDB:3T6A) onto the complex structure of the BCAR1 and NSP3 C-terminal domains (PDB ID 3T6G). The two domains are shown in schematic representation, and residues in the binding interfaces that were mutated are shown in sphere representation (left, BCAR3, orange; right, BCAR1, green). c, effects of BCAR1 and BCAR3 mutations on the association between the two proteins as determined by coimmunoprecipitation (IP) from HEK293 cells transfected with the indicated combinations of empty vector controls, EGFP-BCAR1, and BCAR3 wild-type or mutant plasmids. To avoid possible interference with the BCAR1-BCAR3 association by the immunoprecipitating antibody, BCAR1 was immunoprecipitated with an antibody to the EGFP tag (which was also used for detection of EGFP-BCAR1 by immunoblotting), and BCAR3 was immunoprecipitated with an antibody to an epitope near the N terminus. Cell lysates were also probed, revealing low expression of the BCAR3 L744E and L744E/R748E mutants, which is likely due to their impaired interaction with both wild-type and transfected BCAR1. GAPDH was probed as a loading control. Mutations: 744, L744E; 748, R748E; 787, L787E; 794, F794R. d, transient transfection of BCAR1 in HEK293 cells increases the levels of cotransfected wild-type BCAR3 and different mutations in the BCAR3-interacting domain of BCAR1 impair this effect to different extents. Cells were transiently transfected with empty vector control (V) or with a BCAR3 WT plasmid together with BCAR1 wild-type, L787E (787) or F794R (794) single mutants, or the BCAR1 L787E/F794R double mutant. Cell lysates were probed by immunoblotting with the indicated antibodies, with the immunoblot for GAPDH demonstrating equal loading of the lanes.