FIGURE 10.
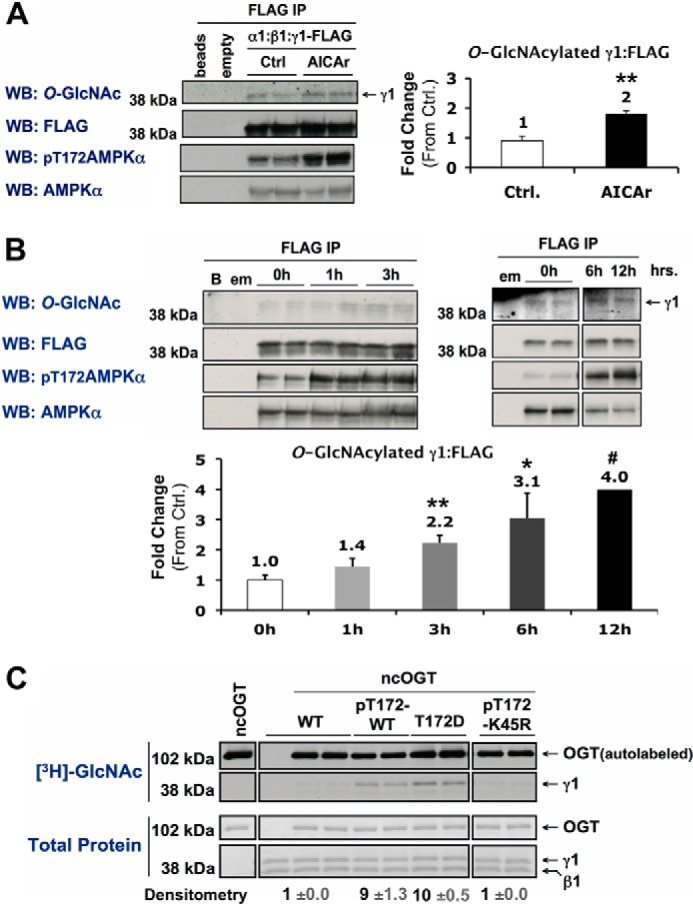
Activation of AMPK increases O-GlcNAcylation of its γ1-subunit. A and B, Myc-α1, β1, and γ1-FLAG constructs were co-expressed in Hek293A cells incubated in vehicle (Ctrl) or AICAr (1 mm, 2 h) (A), or 0 mm glucose for 0, 1, and 3 h or 0, 6, and 12 h (B). AMPK α:β:γ1-FLAG complexes were immunoprecipitated (IP) with anti-FLAG beads and immunoblotted (WB) for O-GlcNAcylated protein and controls as indicated. Anti-FLAG beads incubated without cell lysate (beads or B), and anti-FLAG IPs of cells expressing an empty (α) vector (empty or em) were included as negative controls. C, wild-type (WT), phosphorylated active wild-type (pT172-WT), constitutively active mutant (T172D), or phosphorylated kinase-dead mutant (pT172-K45R) recombinant AMPK α1β1γ1 was incubated with recombinant O-GlcNAc transferase (ncOGT) in the presence of UDP-[3H]GlcNAc (an autofluorograph ([3H]GlcNAc; top panels) of the same gel stained with G250 Coomassie Blue (Total Protein; bottom panels)). Densitometric quantification of O-GlcNAcylated γ1 pixel intensities (mean value ± S.E.) were normalized to respective controls. *, **, and *** denote statistical significance of p < 0.05, p < 0.01, and p < 0.001, respectively. #, 12h time point is representative of one experiment.
