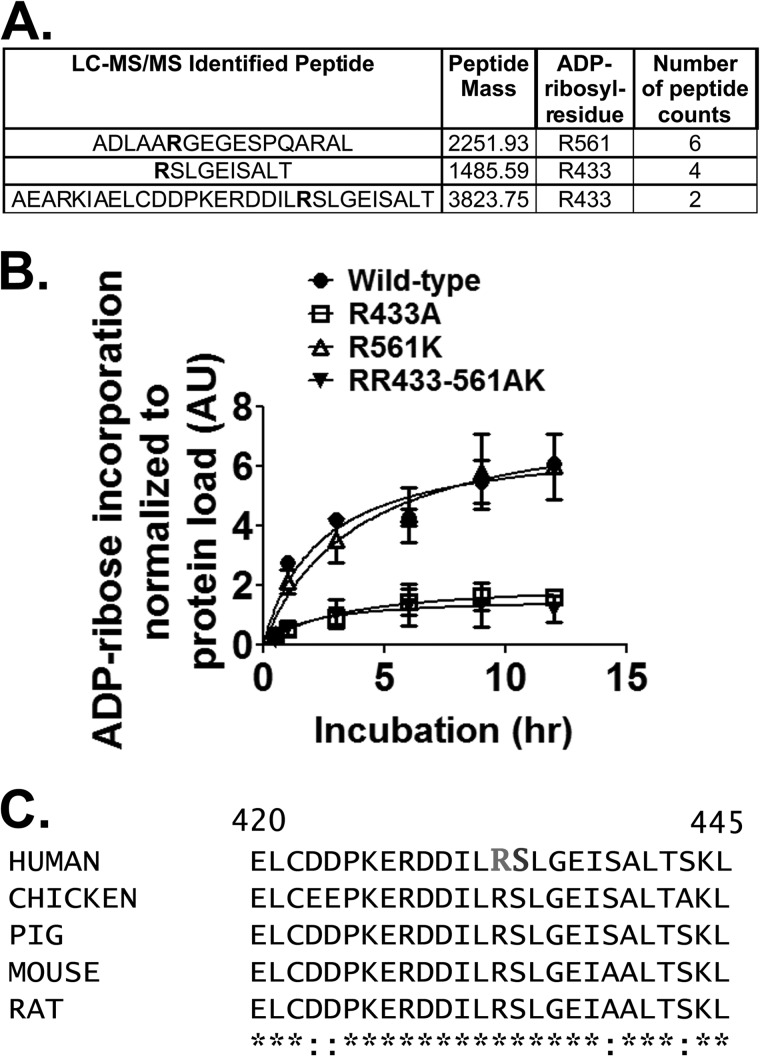
FIGURE 5.
CerADPr ADP-ribosylates vinculin at Arg-433. A, recombinant vinculin was ADP-ribosylated to saturation and subjected to mass spectrometry peptide sequencing. Three candidate arginine residues that had mass increases consistent with addition of ADP-ribose are shown in bold in the peptide sequences. B, site-directed mutagenesis identified Arg-433 as the site of ADP-ribosylation on vinculin. Wild-type vinculin (circles), vinculin(R433A) (squares), vinculin(R561K) (open triangles), and vinculin(R433A-R561K) (closed triangles) were ADP-ribosylated by CerADPr over the indicated time course as described under “Materials and Methods.” Plotted is the amount of ADP-ribosylated vinculin per total vinculin in arbitrary units (AU). C, amino acid sequence of selected vinculin isoforms is conserved. The primary amino acid sequences of several mammalian- and chicken-vinculin (accession numbers: human, AAA61283; chicken, NP_990772; pig, NP_999099; mouse, NP_033528.3; and rat, NP_001100718.1) surrounding Arg-433 and Ser-434 (residues 420–425) are shown. Human vinculin Arg-433 and Ser-434 are in bold.