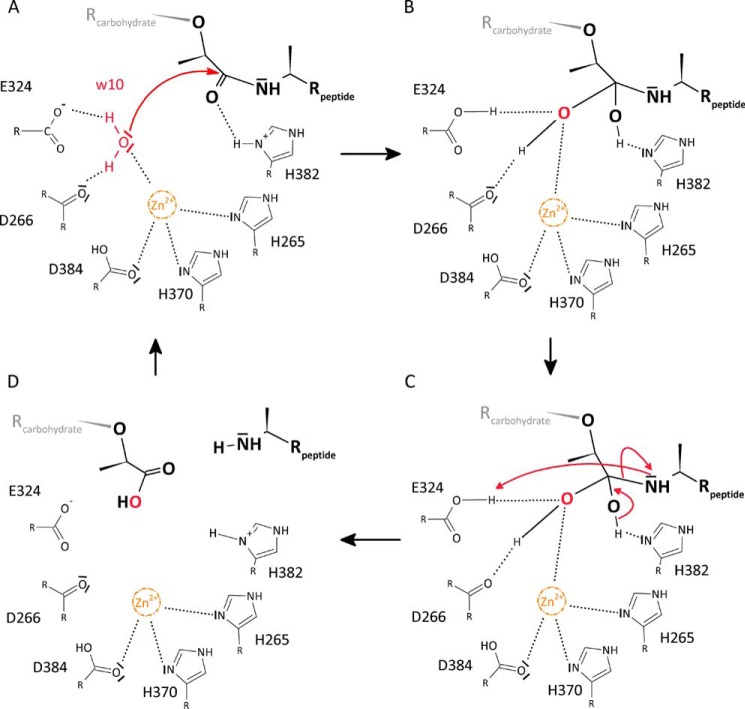
FIGURE 4.
Proposed reaction mechanism of AmiA. A, Wat-10 (w10) is hydrogen-bonded to Asp-266 and Glu-324, and its free electron pairs face toward the scissile bond. Zn2+ is complexed by His-265, His-370, and Asp-384 and probably renders Wat-10 more reactive, enabling a nucleophilic attack. B, tetrahedral intermediate is stabilized by hydrogen bonds of the resulting hydroxyl groups with Nδ of His-382 as well as Asp-266, Glu-324, and zinc, respectively. C, reformation of a carbonyl group with the peptide moiety as leaving group. His-382 can accept a hydrogen atom from the tetrahedral intermediate, whereas the peptide is poised to accept a hydrogen from Glu-324. D, product release.