FIGURE 7.
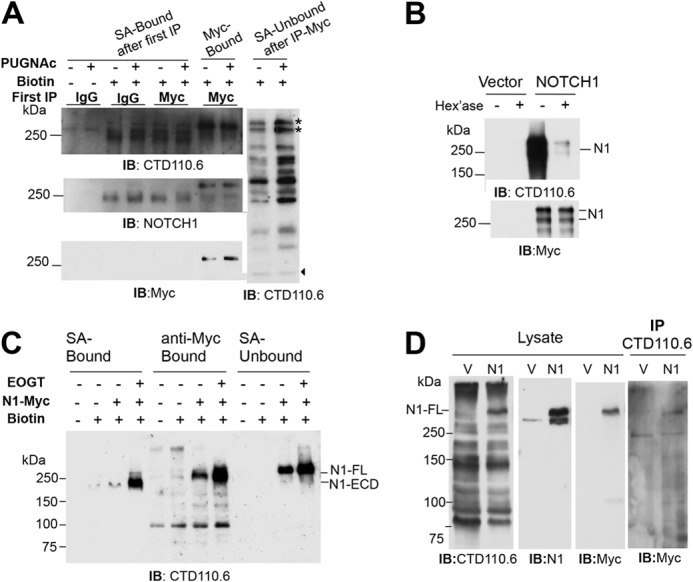
O-GlcNAc on NOTCH1 is detected at the cell surface. A, Lec1 cells transiently expressing NOTCH1-Myc were treated for 18 h with 100 μm PUGNAc (+) or vehicle (dimethyl sulfoxide) (−), biotinylated, lysed, and the lysate was incubated with anti-Myc beads (Myc-Bound) or anti-IgG beads. The fraction that did not bind to anti-Myc or anti-IgG beads was incubated with SA-agarose and gave the (SA-Bound after first immunoprecipitation (IP)) and (SA-Unbound after immunoprecipitate-Myc) fractions. Samples were separated on a 7.5% SDS-polyacrylamide gel, and one set was probed with anti-NOTCH1 ECD mAb (2 μg/ml), stripped, and probed with anti-Myc mAb (1:500). The second set was probed with CTD110.6 (0.4 μg/ml). The stars identify proteins that migrated similarly to but distinct from NOTCH1-Myc. Arrowhead identifies band indicating equal loading. Representative results are from three independent experiments. IB, immunoblot. B, NOTCH1-Myc prepared from transient CHO transfectants as in A was incubated with 10 units of β-N-acetylhexosaminidase at 37 °C for 2 h, separated in a 7.5% SDS-polyacrylamide gel, transferred to PVDF membranes, and incubated with CTD110.6 (0.4 μg/ml) and anti-mouse IgM-HRP (1.6 μg/ml) or anti-Myc 9E10 (1:500) and anti-mouse IgG-HRP (0.08 μg/ml) Abs at room temperature for 1 h. Representative results are from two independent experiments. C, Lec1 cells were co-transfected with vector or pCR3.1/EOGT, and pCS2+/Notch1-Myc. After 24 h, biotinylation was performed; biotinylated proteins were collected on SA-agarose beads or by incubation with anti-Myc antibody and protein G beads, and analyzed by SDS-PAGE and Western blotting with CTD110.6 mAb as in A. D, lysate from Lec1 cells transfected with plasmid pCS2+ (V) or pCS2+/Notch1-Myc (N1) was precleared with anti-IgM-agarose, incubated with mAb CTD110.6 (1 μg) overnight at 4 °C, and collected on mouse anti-IgM-agarose. Lysate (50 μl) and proteins solubilized from beads were separated on a 7.5% SDS-polyacrylamide gel, transferred to membrane, and subjected to Western blot analysis using the indicated antibodies sequentially, in the order CTD110.6, anti-NOTCH1, and anti-Myc antibodies. Representative results are from three experiments.
