FIGURE 2.
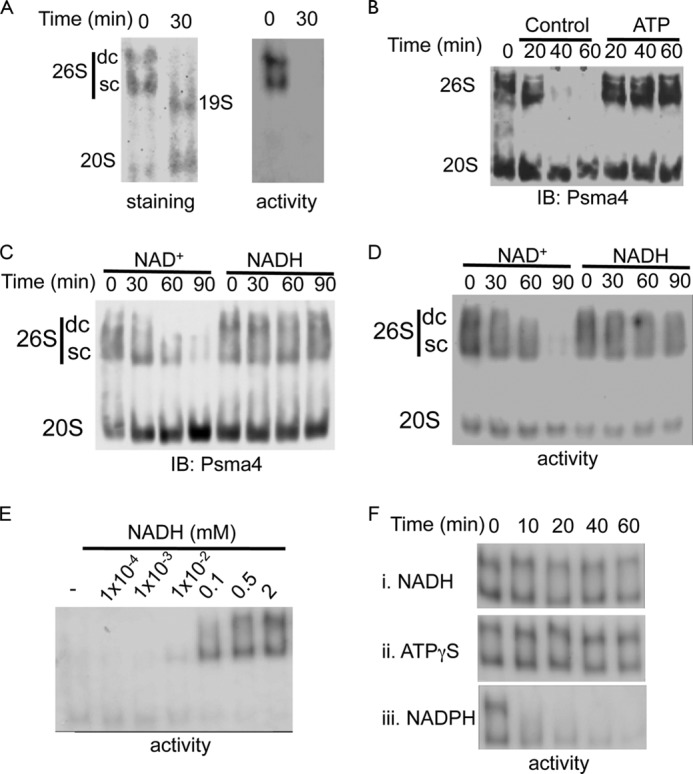
NADH maintains 26S proteasome complex integrity in the absence of ATP. A, purified 26S proteasomes were incubated at 37 °C for 30 min in the absence of ATP, and the 26S PC integrity was analyzed. B, proteasomes fractionated from NIH3T3 cells were incubated at 37 °C for the indicated time points in the absence or presence of 2 mm ATP. C and D, NADH stabilizes the 26S proteasomes. Proteasomes fractionated from NIH3T3 cells were incubated at 37 °C for indicated time in the presence or absence of 2 mm NADH or NAD+. Proteasome complex levels (C) and activity (D) were examined. E. NADH dose dependent stabilization of purified 26S proteasomes. Purified 26S PC were incubated in the presence of 8 milliunits/ml apyrase at 37 °C for 30 min in the presence of increasing concentrations of NADH. Proteasomal activity was analyzed. F, NADH stabilizes the 26S PC in the absence of ATP. In the presence of 8 milliunits/ml apyrase that depleted residual ATP, the purified active 26S proteasomes were stabilized only in the presence of NADH (i) but not NADPH (iii). Incubation with ATPγS (ii) was used as a positive control. SC, single-capped 26S; DC, double-capped 26S.
