FIGURE 1.
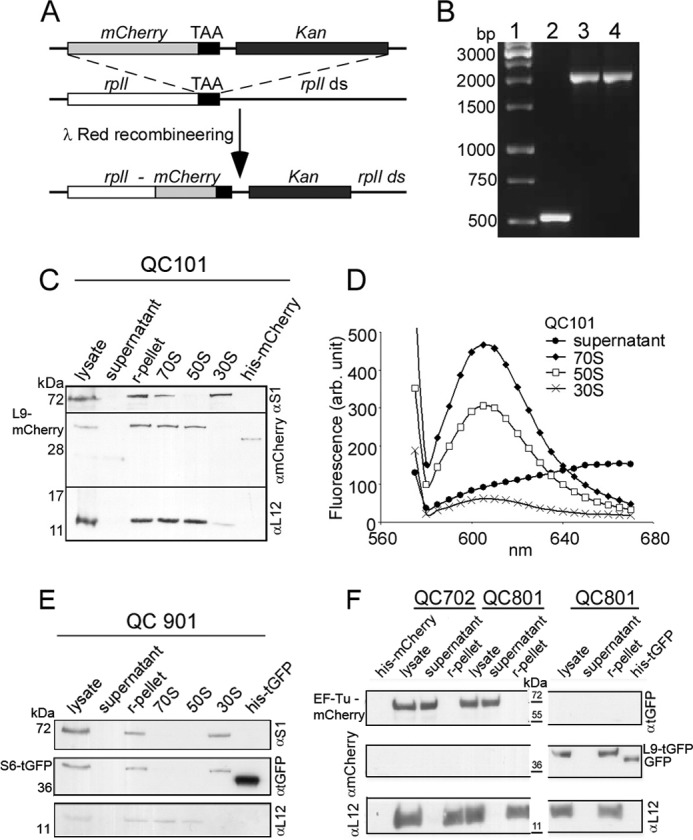
Construction and characterization of E. coli strain QC101 (L9-mCherry), QC901 (S6-TurboGFP), QC702 (EF-Tu-mCherry), and QC801 (EF-Tu-mCherry and L9-TurboGFP). A, scheme showing the strategy for fusion of red fluorescent protein mCherry with ribosomal protein L9. λ-Red recombineering was used to insert a linear DNA containing tandemly arranged genes for mCherry and Kan replacing the stop codon of rplI gene on MG1655 chromosome. The resulting recombinant QC101 produced an in-frame fusion at the 3′-end of rplI gene with the gene for mCherry (see “Experimental Procedures”). B, colony PCR screening for mCherry-Kan (1.5 kb) fusion to rplI (0.5 kb) using primers flanking rplI gene. Lane 1, DNA ladder; lane 2, PCR from colonies of MG1655; lane 3, recombinant QC101 with pSIM5; lane 4, QC101. C, Western blot analysis of cellular fractions from QC101 to trace the L9-mCherry fusion protein. The cell lysate, ribosomal pellet (r-pellet), supernatant (collected after pelleting ribosomes by ultracentrifugation), purified ribosomal particles 70 S, 50 S, and 30 S from QC101, and purified mCherry protein (31.5 kDa) were subjected to Western blot using α-mCherry antibody. Antibodies to the ribosomal proteins S1 and L12 (gift from J. P. Ballesta, CBMSO, Spain) were used to specify 30 S and 50 S subunits. The blot corresponding to L9-mCherry fusion (42.5 kDa) was visible in r-pellet and 70 S and 50 S subunits but not in the supernatant and 30 S subunits. D, fluorescence spectra of 70 S, 50 S, and 30 S ribosomal particles from QC101. Characteristic mCherry fluorescence (excitation at 555 nm and emission λmax 610 nm) was seen in 70 S ribosomes and 50 S subunits but not in the ribosome-free supernatant. Small fluorescence seen in the 30 S subunits could be from minor contamination of 50 S particles. E, Western blot analysis of the cell lysate, ribosomal pellet, ribosome-free supernatant, and the ribosomal particles 70 S, 50 S, and 30 S from QC901 cells separated on a 14% SDS-PAGE using antibodies α-turboGFP, α-S1 (specific to 30 S), and α-L12 (specific to 50 S). Purified TurboGFP (30.5 kDa) was used as a control. Immunoblotting detected S6-turboGFP fusion (40.7 kDa) in the ribosomal pellet and 30 S subunits but neither in the supernatant nor in the 50 S subunits. F, Western blot analysis of cell lysate, ribosomal pellet, and ribosome-free supernatant from QC702 and QC801 cells with α-turboGFP, α-mCherry, and α-L12 antibodies. Purified TurboGFP (∼36 kDa) and mCherry (31.5 kDa) were loaded as positive controls and size markers. In both QC702 and QC801 EF-Tu-mCherry fusion (∼70 kDa) was detected in the lysate and in the supernatant fraction, whereas L9-TurboGFP (∼41 kDa) was present exclusively in the ribosomes of QC801.
