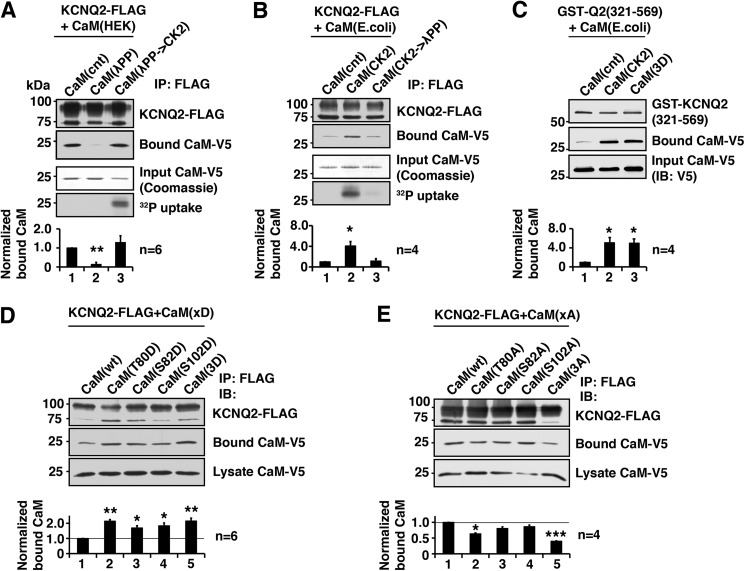
FIGURE 1.
CK2 phosphorylation of calmodulin facilitates binding of calmodulin to the KCNQ2 channel. A, in vitro binding assay showing that dephosphorylation of CaM purified from HEK293A cells by λPP diminished the ability to bind KCNQ2, which was restored by rephosphorylation by CK2. Coomassie Blue staining of input CaM and its autoradiography are shown to demonstrate that CK2 phosphorylated purified CaM. Bottom histogram shows summary of quantification from independent experiments. Corresponding lane numbers are indicated. IP, immunoprecipitation. B, in vitro binding assay showing that CaM purified from E. coli, which lacks endogenous CK2, showed increased binding after CK2 phosphorylation, which was diminished by dephosphorylation by λPP treatment. Protein staining and 32P uptake into CaM are also shown. C, in vitro binding assay showing GST fusion protein containing KCNQ2(321–569) and E. coli generated CaM with indicated CaM treatment and mutation. CK2 phosphorylation and untreated CaM(3D) mutant showed facilitated KCNQ2 binding compared with untreated wild-type CaM. D, immunoprecipitation of KCNQ2-FLAG co-expressed with aspartate substitutions of CK2 phosphorylation sites of CaM. E, immunoprecipitation of KCNQ2-FLAG co-expressed with alanine substitution of CK2 phosphorylation sites. *, p < 0.05; **, p < 0.01; ***, p < 0.001 by nonparametric ANOVA followed by Dunn's multiple comparisons test. Error bars show S.E.