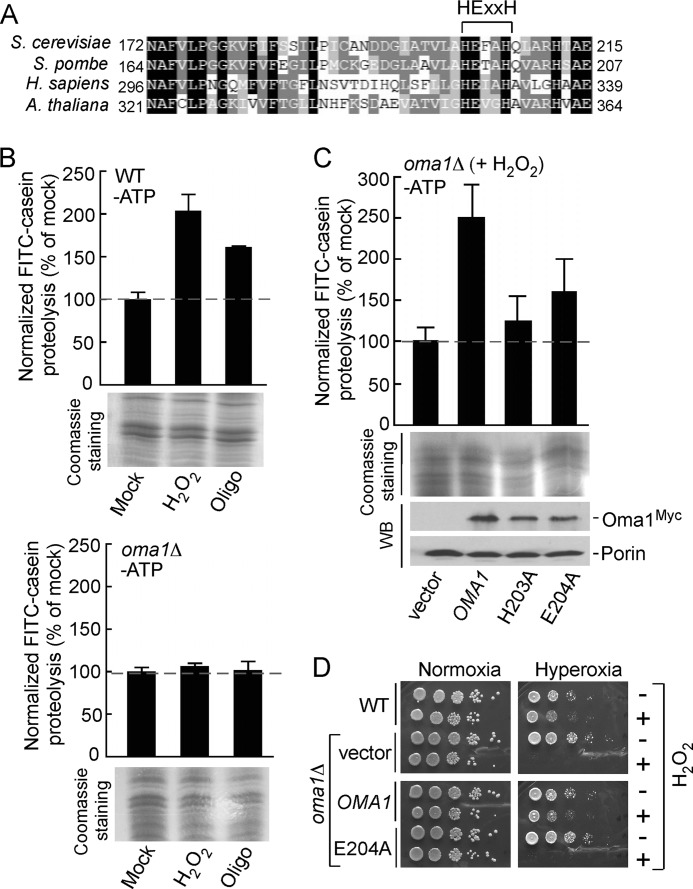
FIGURE 2.
Oma1 proteolytic function is critical for cell survival under stress. A, amino acid sequence alignment of the conserved fragment of yeast Oma1 and its homologs. Sequences were aligned using MultAlin and BoxShade programs. Identical amino acid residues are shaded in black, conserved residues are shaded in dark gray, and the similar residues are shaded in light gray. The signature metalloprotease domain is indicated. B, proteolytic activities of the ATP-depleted mitochondrial membranes derived from WT (top panel) and oma1Δ (bottom panel) strains. Cells in the mid-logarithmic phase were diluted with the fresh culture medium and mock treated or stressed with either 4 mm H2O2 (acute 2-h stress) or 4 μm oligomycin (chronic 8-h stress). Membrane fractions derived from isolated mitochondria were resuspended in the reaction buffer and incubated with FITC-casein at 25 °C for 35 min. Membranes were then removed by centrifugation, and collected supernatants were diluted 10-fold and used in fluorescence measurements. The fluorescence intensities were determined at excitation wavelength of 445 nm and an emission wavelength of 515 nm and normalized to the fluorescence of FITC-casein incubated without membranes. The results shown are the averages of three independent experiments; the error bars indicate S.D. Panels underneath the graphs represent the loading control for each proteolytic reaction and show isolated membrane fractions (25 μg) separated by denaturing electrophoresis and visualized by Coomassie staining. C, proteolytic activities of the membranes derived from H2O2-stressed oma1Δ strains expressing wild type Oma1 or its mutant forms with substitutions in the conserved catalytic residues. Steady-state levels of the expressed constructs were examined by Western blot with anti-Myc antibodies. The levels of the outer mitochondrial membrane protein porin, visualized with anti-Por1, served as a loading control. D, indicated cells were grown to mid-logarithmic phase and incubated with (+) or without (−) 6 mm H2O2 for 2 h at 28 °C. Followed incubation, the samples were serially diluted, plated onto the YPD medium, and incubated under normoxia (21% O2 for 36–48 h) or hyperoxia (95% O2 for 48–72 h) conditions at 25 °C.