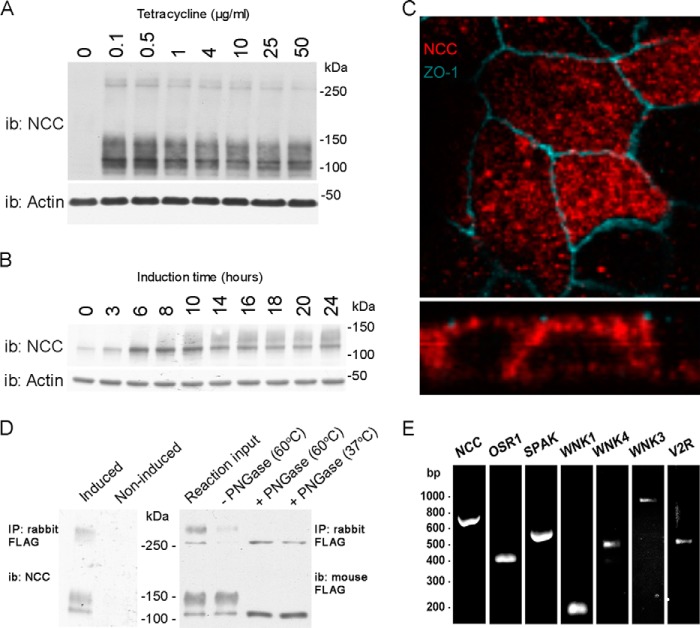
FIGURE 1.
Characterization of a novel inducible MDCKI-NCC cell line. A, representative immunoblot of total protein samples extracted from MDCKI-NCC cells grown until confluent on semi-permeable supports and treated for the last 16 h with various concentrations of tetracycline. NCC is predominantly detected as a diffuse protein band between 100 and 150 kDa using an NCC-specific antibody. Higher molecular weight protein bands (possibly NCC dimers) can be detected above 250 kDa. i.b., immunoblot. B, representative immunoblot of total protein samples extracted from MDCKI-NCC cells grown until confluent on semi-permeable supports and treated with 10 μg/ml tetracycline for various time points. C, confocal laser micrographs (top, xy plane; bottom, xz plane) of MDCKI-NCC cells grown on semi-permeable supports labeled with mouse anti-FLAG (NCC, red) and anti-ZO-1 (blue). NCC labeling is observed intracellularly but also at the level of the tight junction complex. D, left, following immunoprecipitation (IP) of NCC (500 μg of lysate) using a rabbit FLAG antibody, the higher molecular mass bands above 250 kDa are more apparent. Control is noninduced MDCKI-NCC cells. Right, following PNGase treatment, both the higher and lower molecular weight protein bands are reduced in size, confirming that NCC exists as a complex glycosylated protein in MDCKI-NCC cells. Temperatures refer to those used for denaturation of the protein sample prior to PNGase treatment. E, expression of NCC and various genes involved in NCC regulation in MDCKI-NCC cells grown on semi-permeable supports. Conventional RT-PCR analysis of RNA extracted from MDCKI-NCC cells with primers specific for NCC, OSR1, SPAK, WNK1, WNK4, WNK3, and V2R.