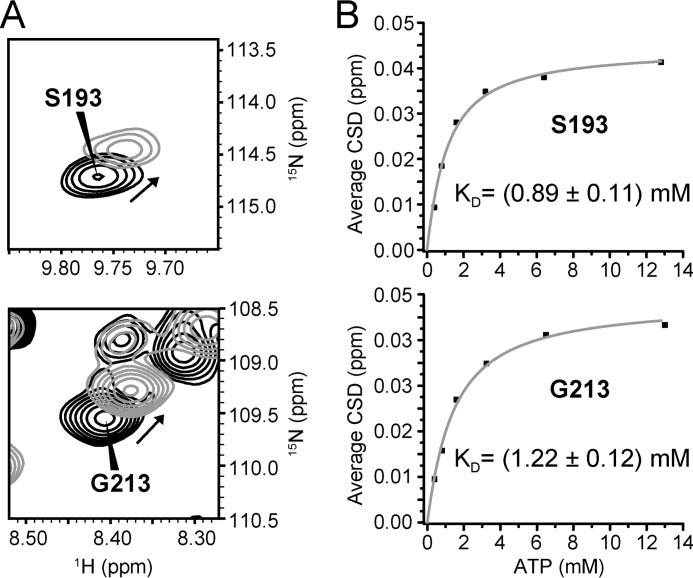
FIGURE 3.
Affinity of hVDAC1 for ATP. A, spectral regions of 1H,15N TROSY spectra before (black) and after (gray) addition of a 32-fold excess of Na2ATP showing examples of residues shifting strongly in the presence of ATP. B, plot of the averaged CSD values versus ATP concentration and curve fits for KD determination using a one-site binding model. Resonance intensity ratios, scaled with respect to apparently unaffected residues (residues 142–145, 152–156, 168–169, and 171–174), were 0.82 (Ser-193) and 1.0 (Gly-213) for the last titration step, showing that these residues were mostly in the fast exchange regime on the NMR time scale. Due to potential contributions from intermediate exchange (41), these values provide lower limits of the KD values.