FIGURE 9.
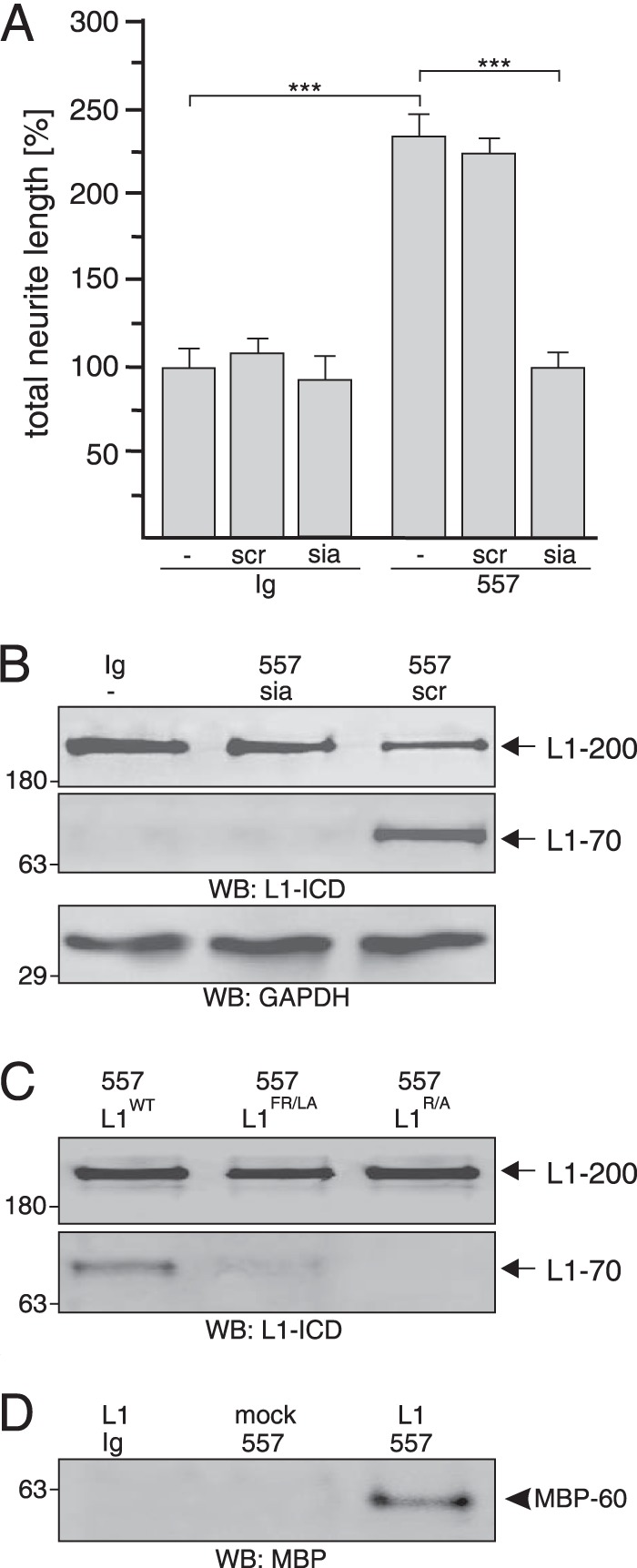
Extracellular MBP cleaves L1 at Arg687. A and B, cerebellar neurons were treated with control antibody (Ig) or L1 antibody 557 in the absence (−) or presence of the L1-derived peptide siabind (sia) or a scrambled version of siabind (scr). Mean values ± S.E. (***, p < 0.001; two-tailed Student's t test) for neurite lengths from three independent experiments (A) and Western blot (WB) analysis of cell lysates with L1 antibody 172-R (L1-ICD) and GAPDH antibody (B) are shown. C, lysates of HEK293 cells transfected with wild-type L1 (L1WT), L1R/A mutant, or L1FR/LA mutant and treated with L1 antibody 557 were subjected to Western blot analysis with L1 antibody 172-R. D, immunoprecipitates with pan-MBP antibody from cell culture supernatants of mock- or L1-transfected HEK293 cells treated with L1 antibody 557 or control antibody (Ig) were subjected Western blot analysis with a MBP antibody specific for the exon II-encoded domain. B–D, representative Western blots of three experiments with identical results are shown. L1–200 and L1–70 are indicated by arrows, and the 60-kDa MBP form is depicted by an arrowhead.
