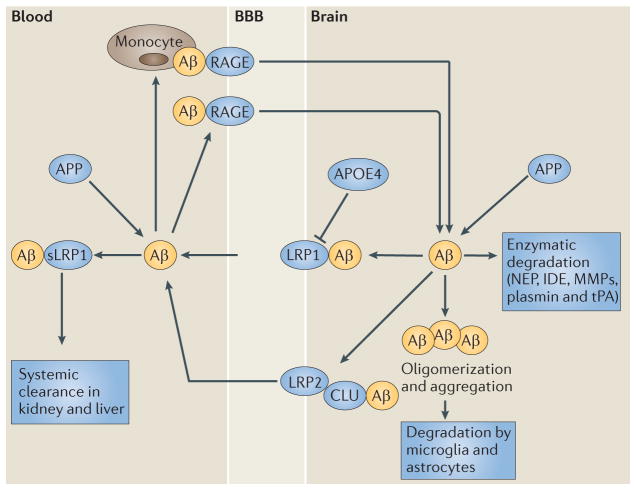
Figure 4. The role of blood–brain barrier transport in brain homeostasis of amyloid-β.
Amyloid-β (Aβ) is produced from the amyloid-β precursor protein (APP), both in the brain and in peripheral tissues. Clearance of amyloid-β from the brain normally maintains its low levels in the brain. This peptide is cleared across the blood– brain barrier (BBB) by the low-density lipoprotein receptor-related protein 1 (LRP1). LRP1 mediates rapid efflux of a free, unbound form of amyloid-β and of amyloid-β bound to apolipoprotein E2 (APOE2), APOE3 or α2-macroglobulin (not shown) from the brain’s interstitial fluid into the blood, and APOE4 inhibits such transport. LRP2 eliminates amyloid-β that is bound to clusterin (CLU; also known as apolipoprotein J (APOJ)) by transport across the BBB, and shows a preference for the 42-aminoacid form of this peptide. ATP-binding cassette subfamily A member 1 (ABCA1; also known as cholesterol efflux regulatory protein) mediates amyloid-β efflux from the brain endothelium to blood across the luminal side of the BBB (not shown). Cerebral endothelial cells, pericytes, vascular smooth muscle cells, astrocytes, microglia and neurons express different amyloid-β-degrading enzymes, including neprilysin (NEP), insulin-degrading enzyme (IDE), tissue plasminogen activator (tPA) and matrix metalloproteinases (MMPs), which contribute to amyloid-β clearance. In the circulation, amyloid-β is bound mainly to soluble LRP1 (sLRP1), which normally prevents its entry into the brain. Systemic clearance of amyloid-β is mediated by its removal by the liver and kidneys. The receptor for advanced glycation end products (RAGE) provides the key mechanism for influx of peripheral amyloid-β into the brain across the BBB either as a free, unbound plasma-derived peptide and/or by amyloid-β-laden monocytes. Faulty vascular clearance of amyloid-β from the brain and/or an increased re-entry of peripheral amyloid-β across the blood vessels into the brain can elevate amyloid-β levels in the brain parenchyma and around cerebral blood vessels. At pathophysiological concentrations, amyloid-β forms neurotoxic oligomers and also self-aggregates, which leads to the development of cerebral β-amyloidosis and cerebral amyloid angiopathy.