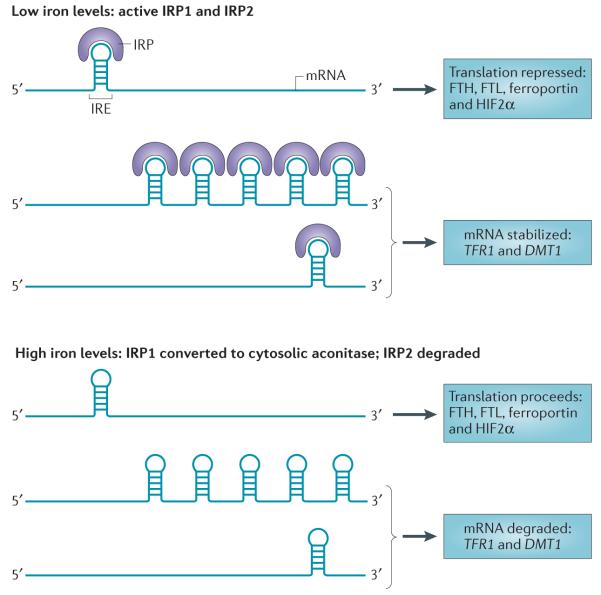
Figure 4. Control of cellular iron metabolism by the IRE–IRP regulatory axis.
Iron-regulatory protein 1 (IRP1) and IRP2 are crucial proteins in the maintenance of cellular iron homeostasis. These proteins bind to iron-response elements (IREs) present in either the 5′ or the 3′ untranslated region (UTR) of mRNAs. IREs are found in the 5′ UTR of mRNAs encoding the ferritin heavy chain (FTH) and ferritin light chain (FTL) subunits, ferroportin and hypoxia-inducible factor 2α (HIF2α), and in the 3′ UTR of mRNAs encoding transferrin receptor 1 (TFR1) and IRE-containing isoforms of divalent metal transporter 1 (DMT1). Binding of IRPs to 5′ IREs inhibits translation, whereas binding to 3′ IREs stabilizes mRNA. IRPs bind to IREs under conditions of low iron levels; under conditions of high iron levels, IRP1 loses its IRE-binding activity and acquires enzymatic activity as a cytosolic aconitase, whereas IRP2 is degraded. Thus, under conditions of low iron levels, the IRE–IRP system functions to increase iron uptake (by stabilizing mRNAs that encode TFR1 and presumably IRE-containing isoforms of DMT1) and decreases iron storage and efflux (by inhibiting the translation of ferritin and ferroportin). Binding of IRPs to mRNAs encoding HIF2α may function as a feedback loop to inhibit erythropoiesis when iron levels are low171.