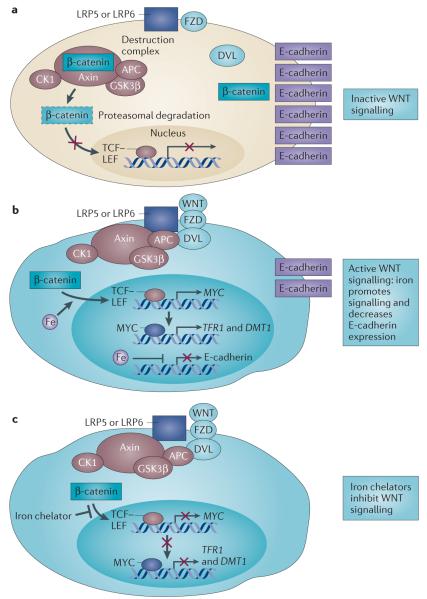
Figure 6. A role for iron in canonical WNT signalling.
a | In the absence of a trigger for WNT signalling, β-catenin associates with axin, adenomatous polyposis coli (APC), casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β) (collectively known as the destruction complex) and is targeted for degradation (shown by dashed outline). Simultaneously, β-catenin is sequestered from the destruction complex and associates with E-cadherin. b | In cells with constitutive canonical WNT signalling (such as cells with mutant APC), β-catenin evades destruction, enters the nucleus and promotes T cell factor (TCF)–lymphoid enhancer factor (LEF)-dependent transcription of downstream target genes such as MYC. Iron promotes TCF–LEF-dependent transcription in cells with such constitutive WNT signalling, resulting in the induction of MYC expression. MYC in turn transcriptionally induces transferrin receptor 1 (TFR1) and divalent metal transporter 1 (DMT1) to promote iron uptake. Iron also decreases E-cadherin mRNA and protein levels in an APC-independent manner. c | Iron chelators decrease TCF–LEF signalling in cells with constitutive WNT signalling at a step distal to β-catenin by an unknown mechanism. DVL, disheveled homologue; FZD, frizzled homologue; LRP, low-density lipoprotein receptor-related protein.