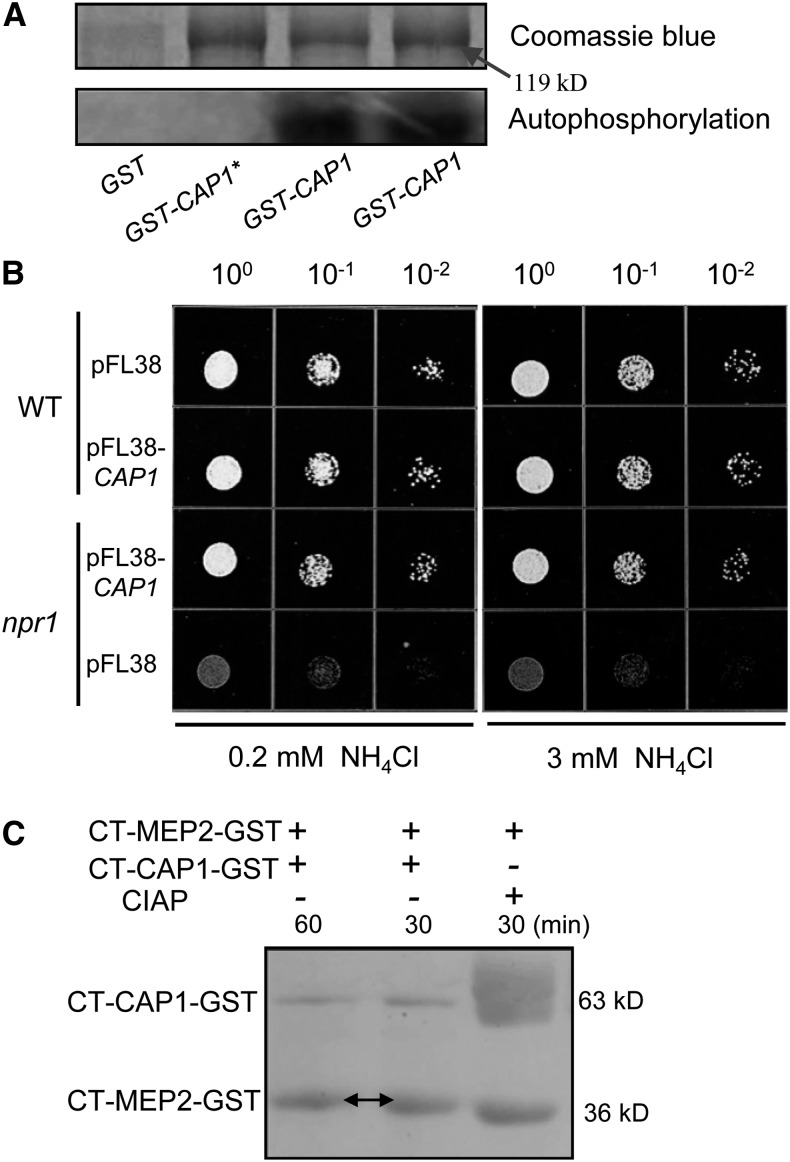
Figure 6.
CAP1 Can Be Autophosphorylated and Complements the Activity of NPR1 Kinase in Yeast.
(A) In vitro phosphorylation of CAP1 kinase activity. Recombinant protein fused with GST was used in the kinase assay. Boiled CAP1 protein (GST-CAP1*) was a negative control. Autophosphorylation was detected after protein gel electrophoresis and phosphor imaging.
(B) Growth of yeast strains on solid minimal medium containing different concentrations of NH4+ as the sole nitrogen source. Strains 23344c (ura3, wild type) and 21994b (npr1-1, ura3) transformed with plasmid pFL38 and pFL38-CAP1 were spotted at 100-, 10−1-, and 10−2-fold dilutions on YNB medium supplemented with 0.2 and 3 mM NH4Cl and incubated for 4 d at 29°C.
(C) In vitro kinase assay for MEP2 phosphorylation by CAP1. Recombinant C-terminal fragments of CAP1 (CT-CAP1-GST) and MEP2 (CT-MEP2-GST) were cultured in kinase buffer, and phosphorylated MEP2 exhibited slower mobility (arrows). MEP2 treated with CIAP (alkaline phosphatase) was the control. The phosphorylation reactions were stopped by boiling for 5 min, and the proteins were separated by SDS-PAGE.