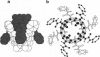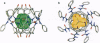Abstract
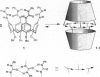
A calix[4]arene was designed to reversibly dimerize and form an egg-shaped enclosure. Adhesive interactions in the assembly were provided by four self-associating ureas, which form a cyclic array containing 16 hydrogen bonds. The synthesis was completed in four steps from the previously described O,O',O",O"'-tetrabenzylcalix[4]arene. Evidence for dimerization of the calixarene tetraurea was provided by H NMR, mass spectrometry, and the observation of encapsulated molecules. The resulting cavity was of sufficient size to capture guests such as ethyl benzene and p-xylene.
Full text
PDF
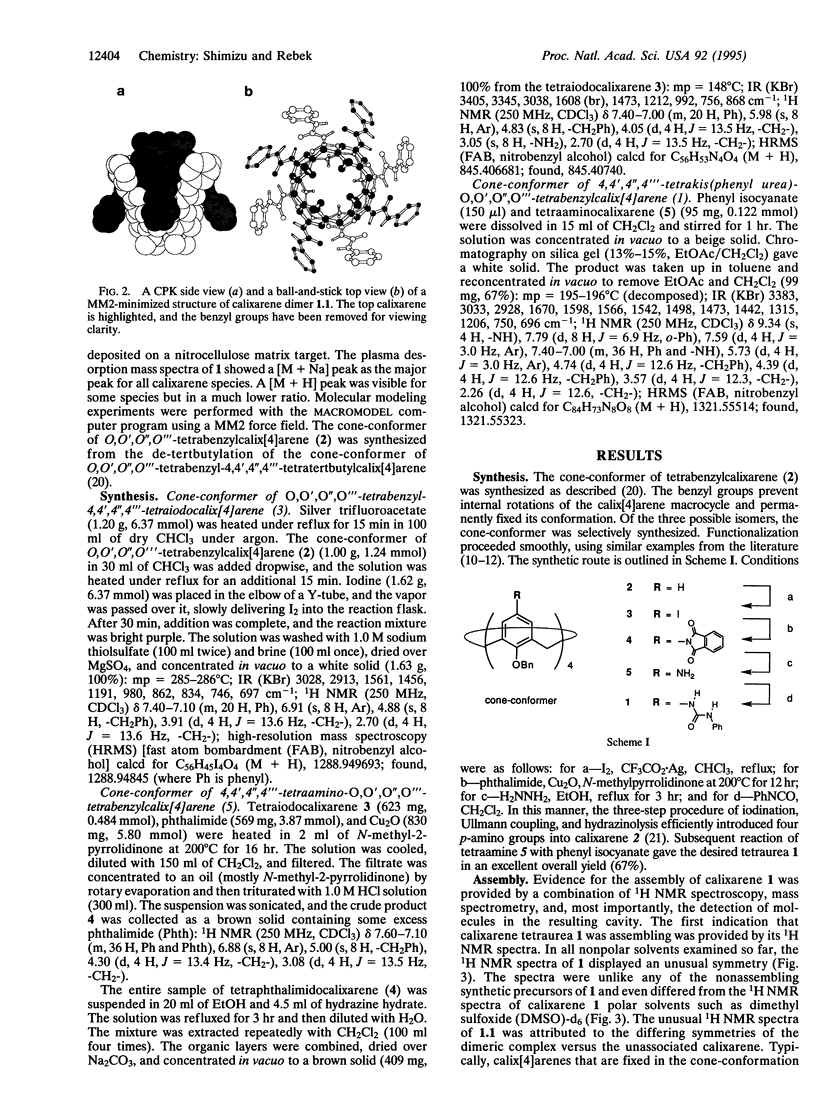
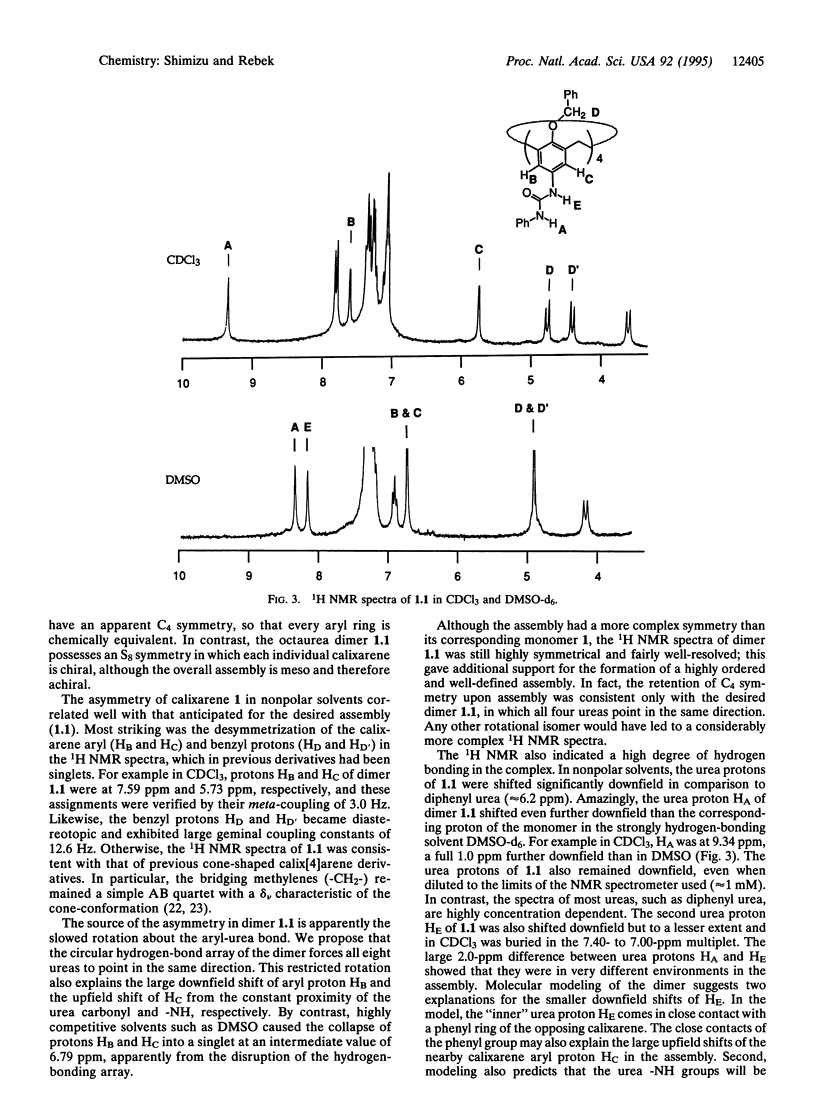
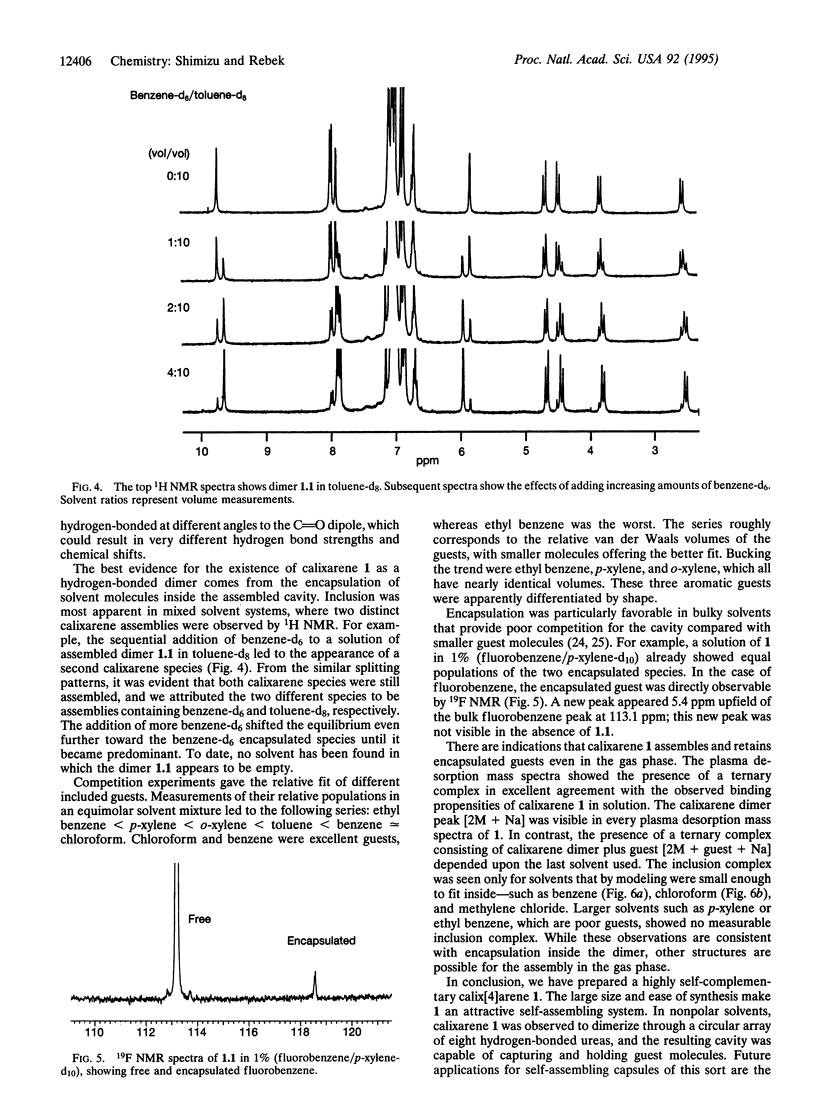
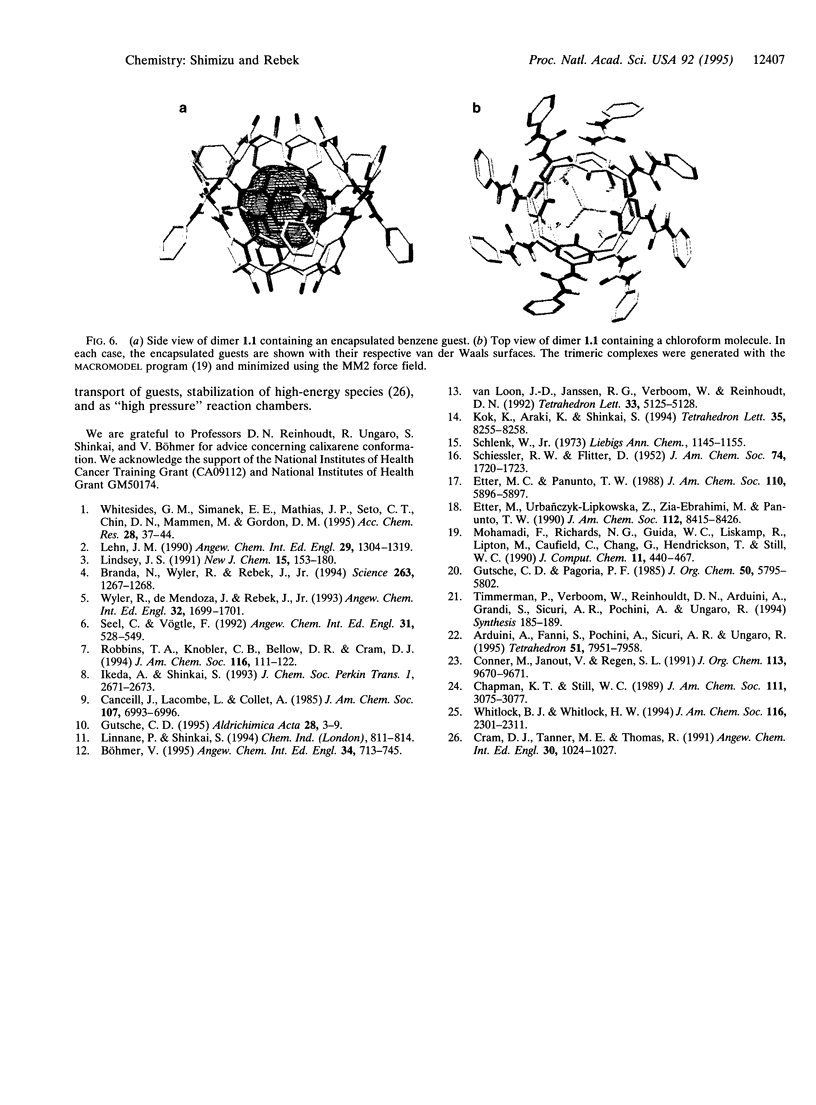
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branda N., Wyler R., Rebek J., Jr Encapsulation of methane and other small molecules in a self-assembling superstructure. Science. 1994 Mar 4;263(5151):1267–1268. doi: 10.1126/science.8122107. [DOI] [PubMed] [Google Scholar]
- Epstein J. B., Mathias R. G., Bridger D. V. Survey of knowledge of infectious disease and infection control practices of dental specialists. J Can Dent Assoc. 1995 Jan;61(1):35-7, 40-4. [PubMed] [Google Scholar]