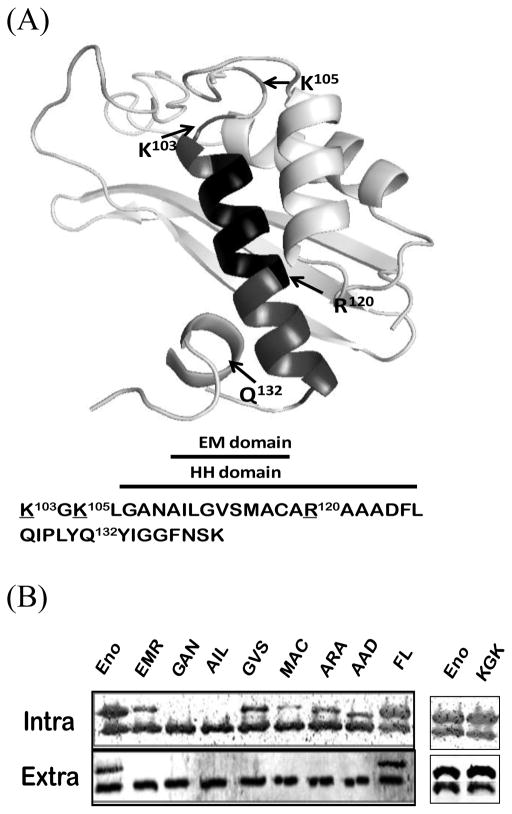
Figure 1.
Predicted ribbon structures of N-terminal domain of Bacillus enolase and the importance of HH domain on enolase secretion. (A). Top panel: Predicted structures of the 140 aa N-terminal domains Bacillus enolase using the Swiss-Plot [15]. The HH α-helix (#106–126) and EM (#110–118) domains are highlighted. Three basic residues (K103, K105, R120), and a small loop replacement (Q132) around HH domain are indicated. (B). Mutations in the HH domain affected the secretion of enolase. Different glycine-substituted mutants were expressed in B. subtilis WB600BHM. Equal volumes of soluble whole-cell lysate (Intra) and supernatant (Sup) fractions were examined by immunoblots. EnoBs antibodies detected both chromosomal wild-type (which were used as internal controls) and plasmid FLAG-tagged enolase (higher band, confirmed with FLAG antibodies). Eno: wild-type enolase; EMR: EM domain replaced enolase [1]; all other mutations are glycine replacements as described in Materials and Methods.