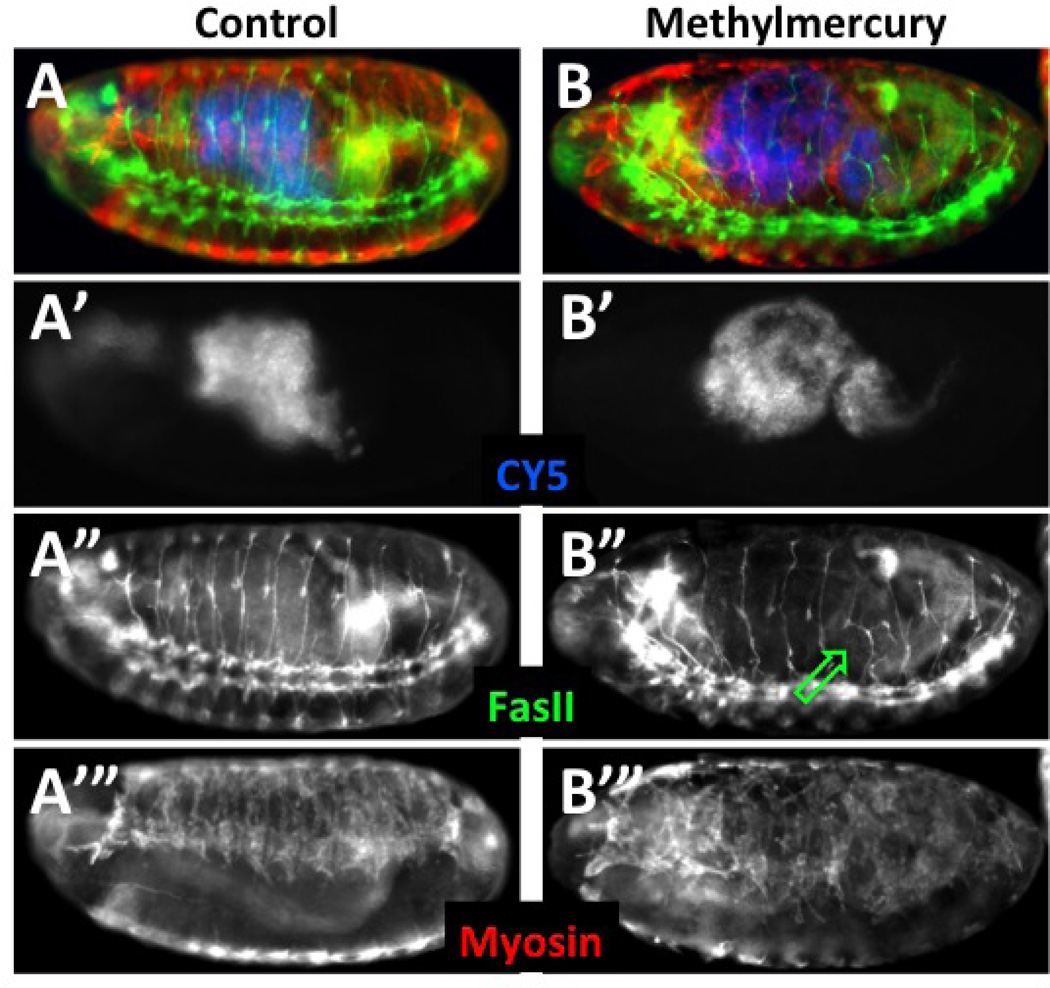
Figure 3. Post-fixation determination of toxin effects in permeabilized embryos.
Embryos were collected and aged at 18°C to obtain a brood of ~stage 8 embryos. At which time they were permeabilized according to Protocol 2 and treated with CY5 dye. Embryos were then developed in notched baskets with either MBIM medium alone or with the addition of 50µM methylmercury and development continued at room temperature for eight hours. Embryos were then fixed and stained as in Protocols 3.1 and 3.2. Embryos that were permeabilized at the outset are identified by direct detection of CY5, which localizes to the lumen of the gut (A’ and B’, pseudo-colored blue in A and B). Segmental and intersegmental motor neurons are stained with anti-FasII antibody (A” and B”, green in A and B) and somatic muscle revealed with anti myosin heavy chain antibody (A’” and B’”, red in A and B). Embryos are equally permeablized as seen by the levels of CY5 dye (A’, B’). Methylmercury is seen to disrupt normal motor neuron projections in the posterior abdominal segments (B”, green arrow). Patterning of developing muscles is similarly seen to be affected strongly by methylmercury (B’” compared to A’”).