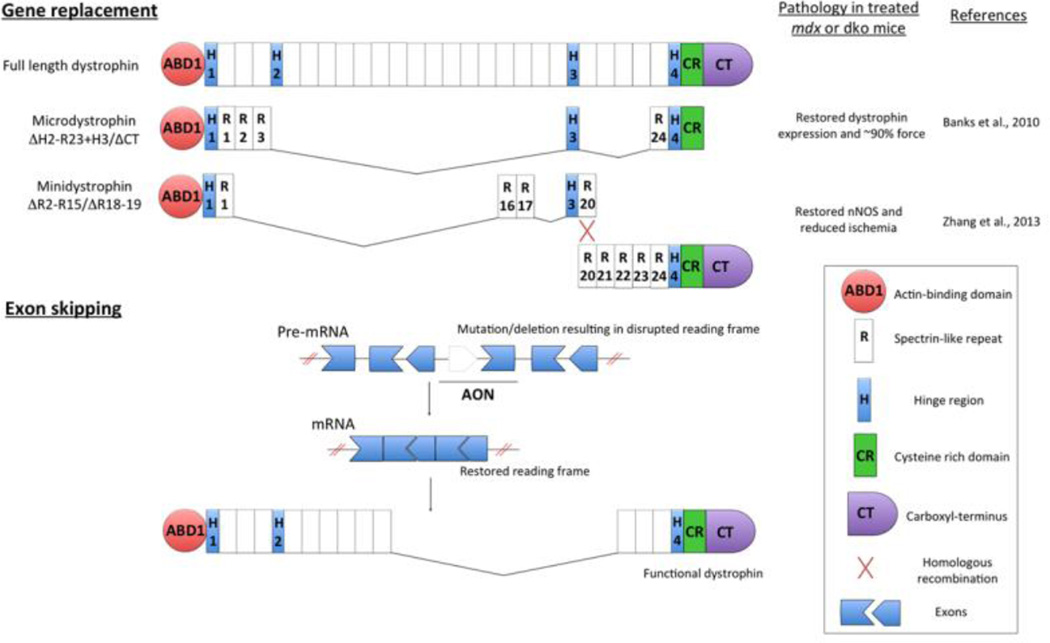
Figure.
Functional dystrophin can be restored through rAAV-mediated gene replacement, exon skipping, mutation suppression (not shown), or cell therapies (not shown). For gene replacement, minimally essential regions of dystrophin are removed to fit the limited carrying capacity of rAAV vectors and generate short dystrophin constructs (microdystrophins). Despite the truncation, microdystrophins restored dystrophin protein production and ~90% of strength in dystrophic mice. Larger dystrophin constructs (minidystrophins) that incorporate additional regions necessary for recruiting important dystrophin binding partners (e.g. nNOS) result in even greater physiological improvements. Minidystrophins are delivered in pieces using multiple rAAV vectors and reassembled in muscle cells by various methods such as homologous recombination. In exon-skipping, synthetic antisense oligonucleotides (AON) are specifically designed to anneal to precursor mRNA (pre-mRNA) and alter RNA splicing to restore normal protein open reading frames, resulting in the production of a smaller, but functional dystrophin. These techniques can be used in combination with cell therapies to correct genetic mutations prior to transplantation back into the patient in ex vivo therapies.