Table 2.
Scope of the AROCM Reaction with respect to Cyclobutene Substitution.[a]
| |||||
|---|---|---|---|---|---|
| R1 | R2 | Product | Yield[b] | %Z[c] | ee Z[d] (ee E)[d] |
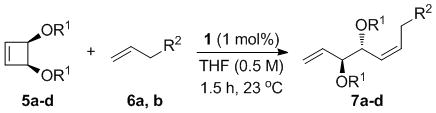
| TBS | OH |
7a
|
66 | 88 | 99 (nd) |
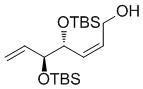
| H | OBz |
7b
|
67 | 75 | 91 (67) |
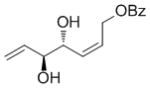
| Bz | OH |
7c
|
69 | 75 | 96 (82) |
| iPr | OBz |
7d
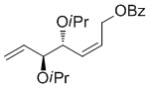
|
<5 | nd | nd |
0.1 mmol cyclobutene, 0.7 mmol terminal olefin.
Combined isolated yield of E and Z products.
Determined by 500 MHz 1H NMR analysis of crude reaction mixture.
Determined by chiral SFC.
