Table 3.
Scope of the AROCM Reaction with respect to Terminal Olefin[a]
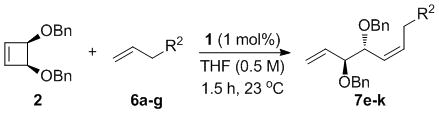
| ||||
|---|---|---|---|---|
| R2 | Product | Yield [b] | % Z[c] | ee Z[d] (ee E)[d] |
| OH |
7e
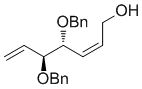
|
62 | 89 | 93 (86) |
| OBz |
7f
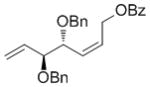
|
61 | 88 | 97 (88) |
| OTBS |
7g
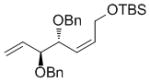
|
68[e] | 87 | 89 (77) |
| OBn |
7h
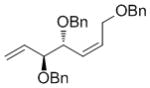
|
64 | 86 | 91 (nd) |
| 4-MeOPh |
7i
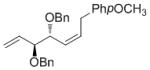
|
76 | 90 | 93 (79) |
| CH2C(O)CH3 |
7j
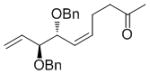
|
65 | 90 | 92 (84) |
| BPin |
7k
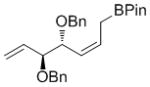
|
50 | nd[f] | 91 (nd) |
0.1 mmol cyclobutene, 0.7 mmol terminal olefin.
Combined isolated yield of E and Z products.
Determined by 500 MHz 1H NMR analysis of crude reaction mixture.
Determined by chiral SFC.
Yield determined after derivatization to 7e.
Not determined due to instability of E product.
