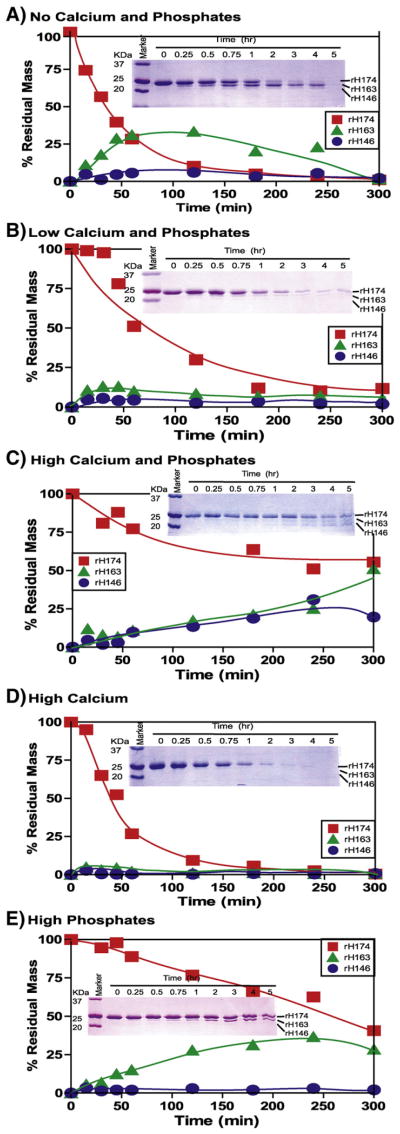
Fig. 1.
Time-dependent proteolytic course of amelogenin (rH174) by MMP-20 at different mineral ion compositions. The substrate (rH174) (50.5 μM) was cleaved by MMP-20 (0.16 μM) and during the time course the substrate and products were visualized by SDS-PAGE (inset) and their quantities are plotted against time. Reactions were carried out in 10 mM Tris buffer, 50 mM KCl, 1 μM ZnCl2, and 60 μM CaCl2 at pH 7.4 with the addition of CaCl2 and KH2PO4. (A) No Calcium and Phosphates – no extra CaCl2 or KH2PO4 added, except 60 μM CaCl2 already present in buffer; (B) Low Calcium and Phosphates – 3.34 mM CaCl2 and 2.09 mM KH2PO4; (C) High Calcium and Phosphates –33.4 mM CaCl2 and 20.9 mM KH2PO4; (D) High Calcium – 33.4 mM CaCl2; (E) High Phosphates – 20.9 mM KH2PO4.