Fig. 1.
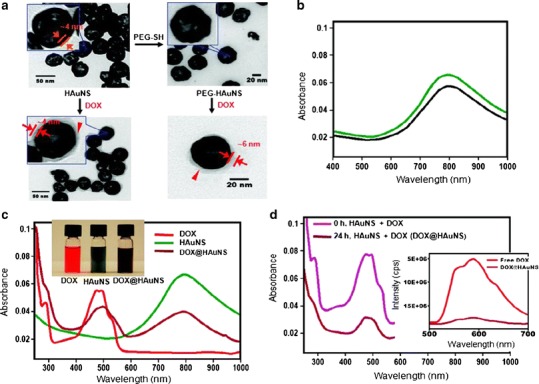
Physico-chemical characteristics of DOX-loaded HAuNSs. a Transmission electron microscope images depicted DOX covering the surface of both DOX@HAuNS and DOX@PEG-HAuNS, which did not exist in HAuNS and PEG-HAuNS before DOX coating. b Ultraviolet-visible absorption spectra exhibited that the plasmon resonance peak of PEG-HAuNSs (black line) did not change as compared with bare AuNSs (green line). c The color of the HAuNS changed from greenish to henna upon absorption of DOX. DOX@HAuNS displayed an ultraviolet–visible absorption peak at 490 nm, which is characteristic of DOX, and a broad NIR plasmon absorption peak at ∼800 nm, which is characteristic of HAuNS. d At 24 h after mixing, the absorbance peak intensity of DOX in the ultraviolet–visible region was significantly reduced compared with the absorbance peak intensity immediately after mixing DOX and HAuNS (0 h) owing to the quenching effect. In addition, compared with free DOX, which exhibited strong fluorescence emission, the fluorescence signal from DOX in DOX@HAuNS was almost completely quenched (d, inset). These results indicate that DOX was tightly bound to HAuNS and PEG-HAuNS after 24 h of incubation. Reproduced with permission from ref. (18). Copyright 2010, American Chemical Society (ACS)
