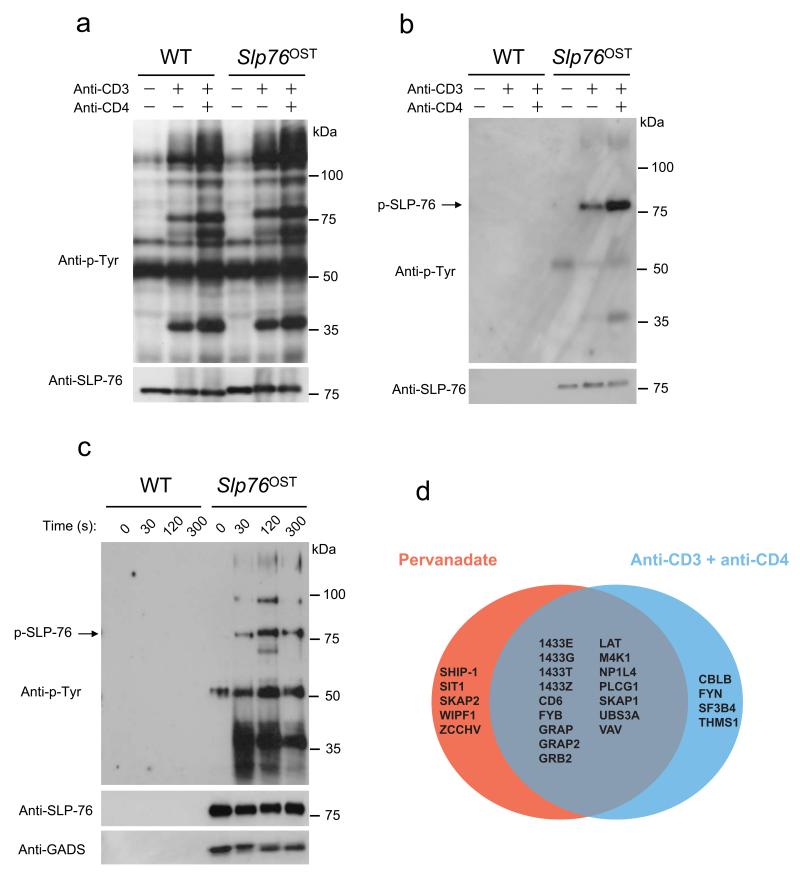
Figure 2.
Purification of SLP-76–associated protein complexes from CD4+ T cells from Slp76OST mice. (a,b) CD4+ T cells from wild-type (WT) or Slp76OST mice were left unstimulated or stimulated for 2 min with anti-CD3 in the presence or absence (key) of anti-CD4. (a) Equal amounts of proteins from total lysates were directly analyzed by immunoblot with anti-phosphotyrosine (Anti-P-Tyr) or anti-SLP-76. (b) Equal amounts of cell lysates were subjected to affinity purification on Strep-Tactin-Sepharose beads and proteins eluted from Strep-Tactin-Sepharose beads with D-biotin were analyzed by immunoblot as in (a). (c) CD4+ T cells from wild-type (WT) or Slp76OST mice were left unstimulated (0) or stimulated with pervanadate for 30, 120 and 300 s and analyzed as in (a). Immunoblot analysis also includes GADS. Data in a-c are representative of at least three independent experiments. (d) Venn diagrams showing the extent of overlap existing between the SLP-76 interacting proteins identified in CD4+ T cells from Slp76OST mice following stimulation with pervanadate or anti-CD3 plus anti-CD4. For more details on the identified high confidence interacting proteins see Supplementary Table 1.