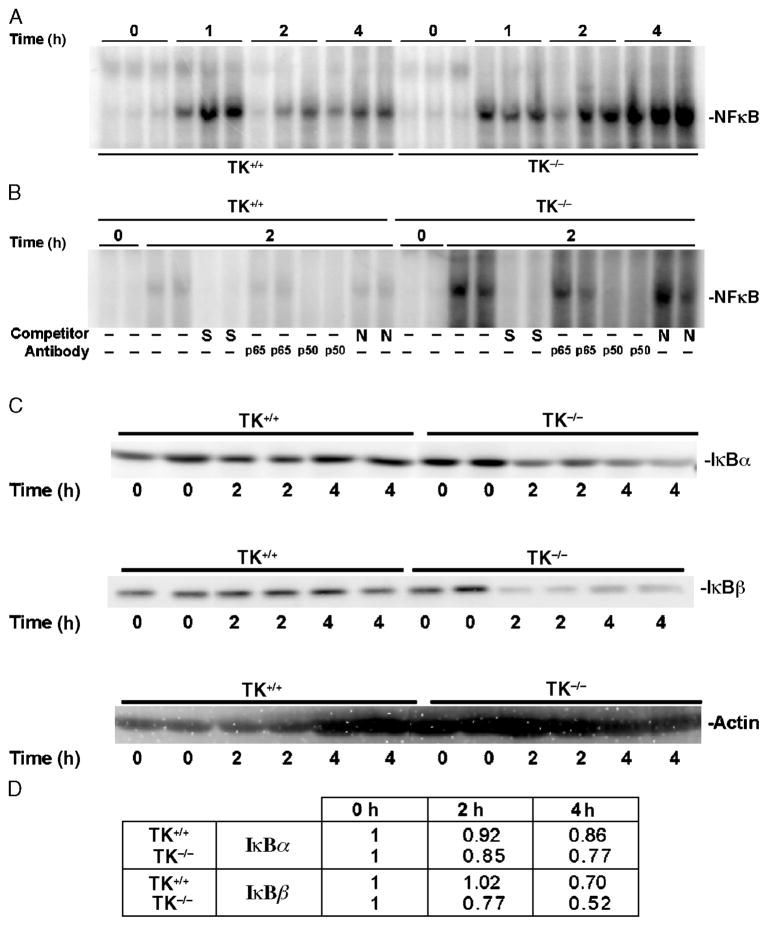
Fig. 3. Effects of Ron signaling on LPS-induced activation of NF-κ B and degradation of I κB proteins.
Whole lung extracts were obtained at 0, 1, 2, or 4 h after intranasal administration of LPS into wild-type (TK+/+) or TK−/− mice. A, Analysis of whole lung nuclear extracts for NF-κB–DNA binding by electrophoretic mobility shift assay. B, Competition and supershift analyses for NF-κB–DNA binding by electrophoretic mobility shift assays. Lung lysates from two independent treatments taken at 0 or 2 h after LPS exposure were used. The protein/DNA complexes were competed with either a 100-fold excess of specific (S) or nonspecific (N) competitor. In addition, antibodies against the p65 or p50 subunits of NF-κB were used. A shift of the specific protein/DNA complexes was observed with antibody addition. No shifts were observed with other antibodies to different NF-κB complexes (data not shown). C, Analysis of IκB protein expression in whole lung lysates. Western blot analysis of IκB-α (top) and IκB-β (middle) protein in TK+/+ and TK−/− lungs. The Western membranes were also reprobed with actin (bottom) to serve as a loading control. Data in A, B, and C are representative of two separate and independent experiments from at least six mice. D, Quantitation of the amount of IκB proteins in C. Densitometry was used to determine the amount of IκB protein. The values represent averages of the two independent samples per time point normalized to the actin control. The values presented are with the 0-h time point normalized to 1.