Figure 1.
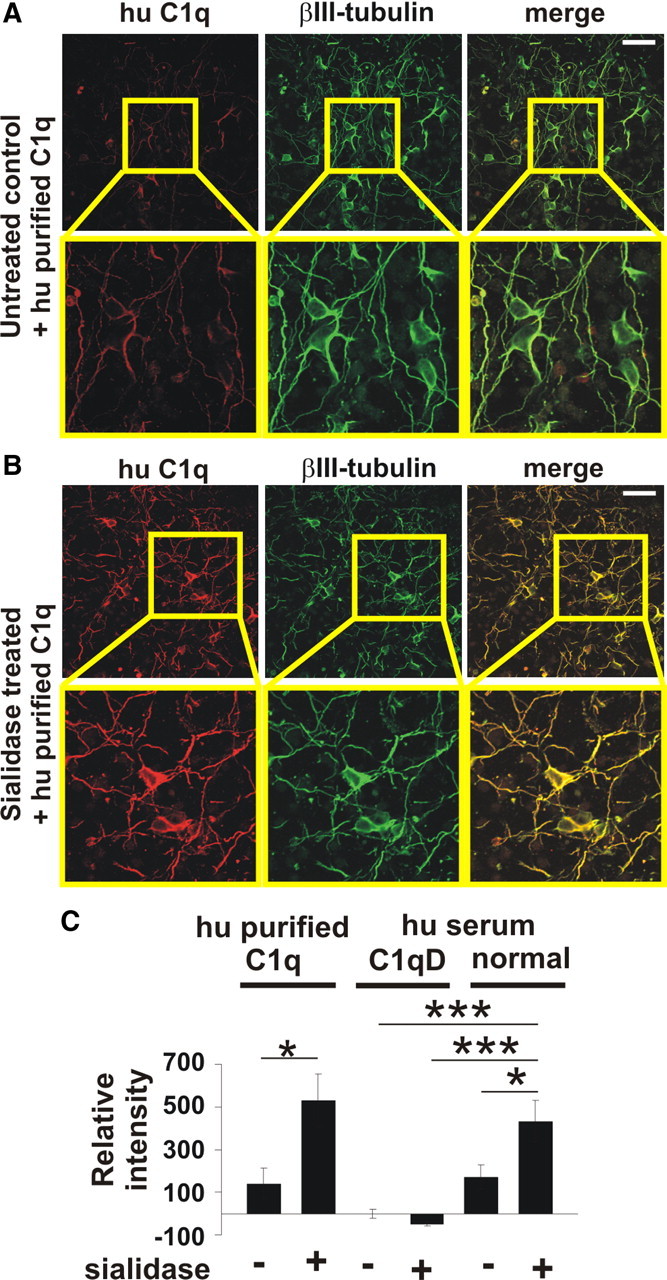
Binding of complement C1 to the desialylated neuronal glycocalyx. Cultured neurons were incubated with human purified C1q (hu purified C1q), serum containing complement (normal hu serum) or serum depleted for C1q (hu serum C1qD). Cells were immunostained with an anti-C1q antibody and double-immunolabeled with βIII-tubulin. A, B, Confocal images of untreated or sialidase-treated neurons. Removal of sialic acids from the glycocalyx led to an increased C1q staining. Scale bar, 50 μm; higher magnification 115 μm, n = 3. C, Quantification of C1 binding to neurons. Purified C1q binding to desialylated neurons was increased compared with untreated neurons (*p = 0.014). Binding of C1 on desialylated neurons was also increased compared with untreated neurons (*p = 0.013), untreated (***p = 1.11 × 10−5) or sialidase-treated (***p = 1.14 × 10−6) neurons incubated in C1qD serum. n = 3.
