Summary
In October 2010, a group of experts met as part of the transatlantic think tank for toxicology (t4) to exchange ideas about the current status and future of safety testing of nanomaterials. At present, there is no widely accepted path forward to assure appropriate and effective hazard identification for engineered nanomaterials. The group discussed needs for characterization of nanomaterials and identified testing protocols that incorporate the use of innovative alternative whole models such as zebrafish or C. elegans, as well as in vitro or alternative methods to examine specific functional pathways and modes of action. The group proposed elements of a potential testing scheme for nanomaterials that works towards an integrated testing strategy, incorporating the goals of the NRC report Toxicity Testing in the 21st Century: A Vision and a Strategy by focusing on pathways of toxic response, and utilizing an evidence-based strategy for developing the knowledge base for safety assessment. Finally, the group recommended that a reliable, open, curated database be developed that interfaces with existing databases to enable sharing of information.
Keywords: nanomaterials, nanotoxicology, alternative methods, 3Rs, Tox-21c
1 Introduction
At the 10th anniversary of the National Nanotechnology Initiative (NNI) and the 2011 NNI Strategic Plan (NNI, 2011), this workshop was organized to evaluate the readiness of the scientific community for developing science-based strategies to assure the safety of the products of this remarkable technology. To accomplish this goal, the Johns Hopkins Center for Alternatives to Animal Testing (CAAT) brought together a diverse group with expertise in toxicology, science policy, regulation, risk assessment, and bioinformatics. This workshop is among the activities of the transatlantic think tank for toxicology (t4), a collaboration of the toxicology oriented Doerenkamp-Zbinden chairs in Konstanz, Utrecht and Baltimore (Daneshian et al., 2010). The t4 activities are sponsored by the Doerenkamp-Zbinden Foundation, Switzerland. Many of the participants were well experienced in research on bioactivity of nanomaterials, applications in nanobiomedicine, and methods for analyzing information. Given the historic commitment of CAAT to the Three Rs (replacement, reduction, and refinement of animal use) and the Center’s new goal of implementing the ideas presented in the 2007 NRC report, Toxicity Testing in the 21st Century: A Vision and a Strategy (Tox-21c) (National Research Council, 2007) regarding the use of animals in toxicology, the participants were encouraged to consider any and all ideas in the course of the three-day discussion.
Over the past five years, publications and research programs in the field of nanotoxicology have increased dramatically. At the same time, the need for science-based strategies for toxicity assessment of nanomaterials has become increasingly urgent. Industry seeks ways to determine which business choices will optimize benefit and minimize risk, as well as provide confidence that toxicity testing will be accepted by regulators. Government needs assurance of transparency and must have a defensible basis for assessment and decision-making, and consumers expect access to information that informs choice and enhances adoption of new products. It is widely recognized that different regulatory agencies have different definitions of assurance of safety or premarket safety assessment requirements. For example, the FDA must have assurance of both safety and efficacy of a drug/device prior to any human exposure in the development and approval process. FDA also requires considerable information on drug/device characterization and manufacturing processes. Each of the statutes that EPA administers has its own requirements for the use of scientific information in considering the risks and/or benefits of chemicals, including nanomaterials. Moreover, assurance may be considered at different stages of the process of evaluating hazard and exposure – for example, in the context of a tiered or integrated approach to nanotoxicity testing, assurance can be defined as a process that will get all stakeholders to agree on nanomaterials or products containing nanomaterials that need public and private resources and attention for higher order and more complex evaluation.
At present, there is no widely accepted path to assure appropriate and effective hazard identification for engineered nanomaterials. Two differing proposals have been made: first, utilization of existing toxicity testing methods to generate a knowledge base covering most endpoints and issues of concern based upon long standing debates in chemical risk assessment; second, the adoption of alternative testing methods to utilize molecular and systems biology as a scaffold for building an integrated testing strategy, reflecting the vision of Tox-21c (Hartung, 2010).
As with industrial chemicals, considering the potential health or environmental impacts of nanomaterials from a Tox-21c perspective will necessitate the development of new testing methods, and strategies for their use, that should ideally be employed prior to the introduction of nano-based materials and products into commerce. At the same time, the field must recognize the increasing need to catch up to the rapid pace of applications already marketed in many countries. “It takes all the running you can do to keep in the same place,” said the Red Queen in Alice’s Adventures in Wonderland. “If you want to get somewhere else, you must run at least twice as fast as that!”
Major topics of discussion by the participants were the necessity, extent, and priority for characterization of nanomaterials. As a rule, one cannot depend upon inferences from studies of bulk materials to predict the likely hazards of nanomaterials – for example, studies on carbon black to infer hazards of carbon nanotubes, of silver ions to evaluate nanosilver, or of larger particles to represent nanoscale materials. This size assumption was challenged by research on ambient particulate matter, which demonstrated that the health hazards associated with air pollution are largely ascribable to smaller natural particles (Brook et al., 2004). The current thinking in nanotoxicology maintains that this inference of biological activity of nanomaterials based on their constituents does not adequately describe the hazards of deliberately engineered nanomaterials, which have distinct characteristics designed into them through advanced methods in chemistry, physics, and materials science. Some of these characteristics may affect key toxicological attributes of the nanomaterial, including its toxicokinetics, toxicodynamics, and its interactions with other materials through co-transport.
Researchers in nanotoxicology are challenged by the diversity and complexity of the materials and their properties now in development, production, and use. We are also aware of the almost complete lack of information on the effects of chronic exposures to these materials as well as the identification and characterization (including agglomeration state, size distribution, shape, surface coating, and release from composites) of those nanomaterials to which people may actually be exposed. Because of the limited state of knowledge of hazard and exposure, it is not clear at present that the taxonomy of nanomaterials is sufficiently developed to define the essential characteristics of all relevant nanomaterials or to predict bioactivity. We are gaining information at a much faster pace than several years ago, however, and in the past year or two there has been a considerable increase in the number of publications containing basic information on potential hazards from some nanomaterials in vivo. Clearly, we are neither at the beginning of understanding the potential health effects, nor are we at the end, having all the answers the public, regulators, and policy makers require. But perhaps we are at the end of the beginning.
2 Characterization of nanomaterials – or not?
The group considered the utility of understanding the novel properties of a nanomaterial in setting priorities for testing, such as redox potential, the generation of reactive oxygen species, ability to enter cells or to change portals of entry, but concluded that, given the lack of knowledge of the key drivers for specific toxicological effects of nanomaterials, it is not appropriate to “rush to judgment” about some characterization measurements as specific signals for prioritization. Given the many variables in characterization of nanomaterials, including purity and stability, as well as the behavior of nanomaterials in biological systems (e.g., agglomeration), it is not clear how to fully ascertain which characteristics of each nanomaterial are the most important to measure when assessing biological activity. Therefore, while it may eventually be possible to develop a predictive system similar to (but distinct from) quantitative structure-activity relationships (QSAR) utilized in assessing chemicals, at present it is not possible to establish such relationships, although there are existing research agendas that strive to achieve development of these predictive models. Even developing an agreed-upon system of annotation of material characteristics may need substantial information about biological activity of nanomaterials before selecting nanomaterial characteristics for annotation and for evaluation. On the other hand, it may be appropriate to generate information about the characteristics of nanoparticles, which, when taken together with the bioactivity information, can be used to interpret the taxonomy of the nanomaterials (for example, to determine how particle size and surface might affect toxicity or absorption, metabolism, distribution and excretion (ADME) data). As an alternative or addition to structure- (material properties) based activity relationships [(Q)SAR], the concept of “biologically-based activity relationships” was discussed. This approach requires testing methods that provide adequate information on the biological activity of nanomaterials. Any structure-or biological-based relationship can only be advanced by the acquisition of appropriately annotated and rigorously curated databases in which a series of related materials are assessed.
The need for guidance on the types of nanotoxicity data required, as well as how to report such data, becomes urgent as the development and introduction of new materials and applications of nanotechnology grow. While it is not possible to stipulate a list of information that would fully represent the data needed on characteristics of nanomaterials being tested (in the absence of a larger set of data), the group recommended some minimal reporting, including the purity and stability of the material being tested, sterility (including testing for endotoxin), a range of different annotations related to dose or amount (including mass, surface area, particle number, and other attributes), assessment of the behavior of the material in the test system (of particular importance in in vitro test systems), and the process by which the nanomaterial was synthesized. Other groups have recommended a uniform system of data reporting; our workshop participants went further to recommend that a single locus of curatorial responsibility should be established. The NCI’s Nano-technology Characterization Laboratory program was cited as an appropriate candidate, given its extensive experience in the testing of nearly 200 nanomaterials for application in medicine and the breadth of its data. Since efforts are also occurring in Europe, the group recommended that data reporting activity in the US be coordinated with that in Europe (for example, through the OECD). (Note: In the interim between the workshop and the publication of this report, the National Heart, Lung, and Blood Institute, the National Institute of Environmental Health Sciences, the National Cancer Institute, and the National Institute of Biomedical Imaging and Bioengineering of the US National Institutes of Health have established a contract with RTI International to develop a curated nanomaterial registry.)
The field of nanotoxicology would also benefit from the availability of standard materials, including standard reference nanomaterials, resource materials for toxicity testing, analytic standards, and the identification of appropriate positive and negative controls. Some discussions on this topic have taken place with NIST; these discussions need to be accelerated and supported.
3 Testing strategies
Considerable uncertainties also limit any recommendations for a set of alternative methods or a fully defined integrated testing strategy for nanomaterials at this time. Nonetheless, the goal expressed in the Tox-21c report – to utilize the concepts of systems biology to assess effects of compounds and materials on pathways of response rather than apical or organ level effects – remains as important to nanotoxicology as to more traditional toxicity testing. Such a strategy is most useful when some information on biological activity is available upon which to base a rational selection of systems and pathways for assessment. While some have proposed that most, if not all, nanomaterials elicit a limited set of responses – inflammation, oxidative stress (or catalyzing the formation of active substances in general), local release of ions – the present lack of comprehensive analysis under conditions of exposure relevant to human health risk assessment must limit confidence that we have truly defined the domains of nanomaterial bioactivity. The use of functional genomics and proteomics tools in nanotoxicology can provide additional data, but the value of this information will be limited as long as it remains disconnected from higher order information.
Evaluating the existing data in nanotoxicology is also hindered by lack of information on current or expected exposures including routes of exposure, form of exposure (e.g., nanoparticles, agglomerates), and amounts, as well as the uptake and distribution of nanomaterials in the body. It is also imperative to understand the behavior of nanomaterials in the environment. Although this workshop deliberately focused more on hazard than on exposure, it was recognized that iterative interactions between these fields is critical to advancing assurance of nanomaterial safety. A balanced integration of hazard and exposure information can also assist in risk management, to facilitate upstream decisions by selecting less hazardous options before production as well as utilizing post-marketing actions to reduce exposure.
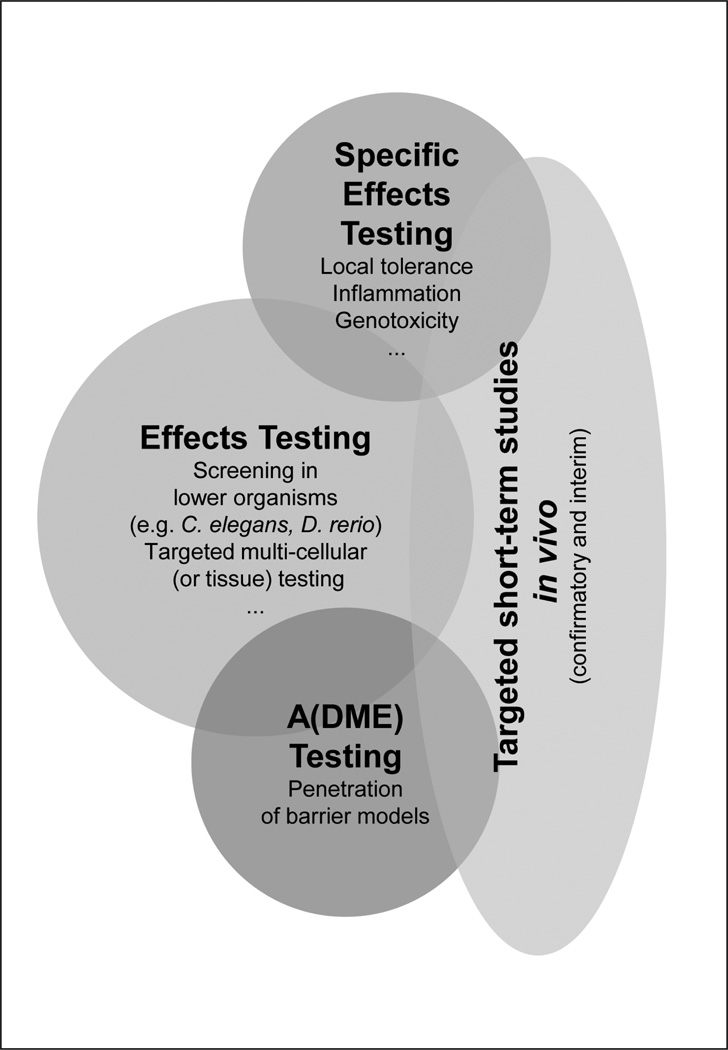
Obtaining information on potential bioactivity, high-throughput, and high-content methods may be useful first steps. Other early methods may include the use of innovative alternative whole animal models, such as zebrafish (Danio rerio) or C. elegans, in which the organism can be monitored during complex stages of development, and perturbations of numerous pathways may be assessed apically. Following these efficient approaches and novel technology, other in vitro or alternative methods can be rationally selected to examine specific functional pathways and modes of action (Fig. 1).
Fig. 1. Schematic diagram incorporating concepts for preliminary assessment of nanomaterial bioactivity.
Some existing tests – such as genetic toxicity protocols – must be evaluated and validated for their suitability in testing nanomaterials.
Figure 1 depicts elements of a potential testing scheme for nanomaterials. Assays for genotoxicity and inflammation as well as skin and eye irritation are examples of alternative methods addressing specific potential effects of nanomaterials. Not all testing can be sufficiently performed using in vitro assays. There is a need to generate reference data for future in vitro testing, however, as well as a need to test today without a complete set of in vitro methods at hand. In fact, many in vitro test systems must be evaluated and validated for sensitive and appropriate responsiveness to nanomaterial challenge. Short-term in vivo rodent assays adapted to detecting the properties of nanomaterials may offer a reduction and refinement approach to more immediate testing needs, although in some instances additional subchronic testing using current guidelines may be necessary.
The group proposes elements of a potential testing scheme for nanomaterials that works towards an integrated testing strategy, incorporates the goals of Tox-21c in focusing on pathways of toxic response, and utilizes an evidence-based strategy for developing the knowledge base for safety assessment.
4 Two thought experiments on approaches to testing
Two thought experiments illustrate how integrated/tier approaches could develop. The first thought experiment involved using selected testing strategies to evaluate the efficacy of current toxicological tests. For instance, if routine assays conclude that a nanomaterial is nontoxic, how can we be sure that this nanomaterial is indeed not toxic? This consideration is important in the context of probabilistic risk assessment because there is still a certain probability that this particular material may be toxic even though it is not showing activity in any of the “classical” assays. Public and regulatory agencies want to minimize the false negatives without the risk of unduly increasing the false positives. To make testing more conclusive and convincing to the public, it was recommended that as part of any tiered strategy one should always take a proportion (e.g., 10%) of the negatives forward to the next tier and to confirm a negative response. This approach would add more confidence to the testing strategy and make the results more reliable.
In a second thought experiment, a group was assigned the task of developing a testing approach for a scenario in which a pharmaceutical company was required to select one from among several candidate nanomaterial-based drugs to submit an application for a new drug approval to the US Food and Drug Administration. The purpose of the exercise was to identify potential issues that might challenge drug development and regulatory submission involving nanomaterials. The scenario was divided into separate dimensions: 1) issues related to identifying the most suitable nanomaterial-enabled drug candidate, and 2) issues related to satisfying requirements for an Investigational New Drug (IND) application, that is, substantiating safety of the proposed product so that the New Drug Application (NDA) phase may begin.
The theoretical new drug candidate was a nanoparticle-based therapeutic product, given intravenously, that destroys prostate cancer cells. The product would consist of an iron-oxide nanoparticle core coated with a polyethylene glycol (PEG) shell with attached ligands (antibodies) developed to bind specifically to prostate cancer cells. Once attached to the cancer cells, radiation would be focused on the cancer cell/drug complex to cause thermo-ablation, with the intent of selectively destroying the malignancy. The FDA would likely consider this product to be a combination drug, medical device, and biologic.
The pharmaceutical product development team identified several potential hypothetical candidate products, and since time and resources were limited, the candidates needed to be screened for safety without using mammalian animal models. A key issue here was the notion that one could not evaluate safety of everything due to a limited budget but that one had a limited set of candidates with which to go forward. A series of tests would be conducted on the candidates. First, the candidate moieties would be incubated with human blood to observe for hemolysis and complement activation (immune response). The stability of the PEG and antibody coating in human blood would need to be evaluated. The new drug candidate could then be cultured in human whole blood for comparison against controls (including existing FDA-approved cancer drugs and FDA-approved biologics, such as antibodies and vaccines). The reasoning behind this approach is that FDA-approved biologics provide a comparably complex entity to nanoparticles, and the FDA has experience evaluating them. Indeed some of the first “nano” drugs are biologics, e.g., protein/drug combinations that are nanoscale in size. By using other biologics as controls or reference materials, concerns about false negatives in non-standard assays might be reduced.
The next test would be done in zebrafish or another low-order, whole animal system such as C. elegans, to compare the candidate products with existing FDA-approved cancer treatment drugs. Other in vitro tests would include treating selected human target and non-target organ cultures with the candidate drugs to assess toxicity and co-cultures of macrophages/epithelial cells to detect phagocytosis potential. For potential efficacy, the nanoparticle-based candidates would be administered to normal and malignant cells derived from prostate to confirm the ability of the drug to adhere to the tumor cells. Finally, since intravenous administration would be in a saline solution, the product would need to be shown to be stable and dispersible in saline.
Investigational New Drug (IND) submissions require data substantiating the safety of the proposed drug in animals, and this process continues once approved for human clinical testing in Phase II studies. The types of testing needed depend, in part, on the intended use, including exposure characteristics. Minimal animal testing would be required to determine dose-range, biodistribution, elimination, and toxicity. A prostate model in the dog may be developed to demonstrate efficacy, as well as safety. The group concluded that the premarket safety assessment envisioned in this case would not be substantially different for a nanoparticle-based therapeutic product than that required for other drugs/biologics.
As is required in all FDA IND or New Drug applications, GMP (Good Manufacturing Practices) must be followed. To this end, information regarding chemistry, manufacturing, and controls (CMC) is required. For chemistry, the chemical properties, composition, physical properties, identity (formula), purity, stability, potency, etc. must be determined. For manufacturing, the company must demonstrate that the product can be produced within defined specifications while also demonstrating product stability, among other attributes. For process controls, the company must provide defined standards and specifications against which the manufacturing process must conform. Characterization of the product would be part of the CMC requirement, and the group concluded that this requirement would be different, unique, and difficult for nanoparticle-based products.
In summary, the group concluded that the FDA IND/NDA submission requirements would be substantially the same for nanoparticles and non-nanoparticle drugs. Safety testing would be similar to the more traditional drug/device/biologic products (e.g., acute and sub-chronic toxicity, reproduction and development, genotoxicity, pharmacokinetics, pharmacodynamics, etc.) depending upon intended use. On the efficacy side, an assay would need to be developed that demonstrates the nanoparticle-complex preferentially adheres to prostate cancer cells in vitro and in vivo.
The GMP requirements, however, and especially CMC, would present significant challenges to a nanoparticle-based product. Given this particular theoretical product, there is no known assay available to confirm that the PEG density is such that it would diminish protein binding by the reticulo-endothelial system attacking the PEG layer and thus diminishing the capacity of the product to bind to the cancer cells. Analytical assays may not be available to characterize and confirm the identity of the therapeutic moiety, as well as demonstrate its stability. Therefore, meeting GMP/CMC requirements and, in particular, characterizing the nanoparticle complex, could be exceedingly difficult. It is likely that such technology does not currently exist.
Overall, the exercise illustrated that although hazard testing would be an important part of the pre-IND process of candidate selection, there is a paucity of available in vitro test methods and data to conduct an adequate safety profile without using traditional animal tests. To this end, there would most likely be little difference in the pre-clinical studies required to substantiate safety between nano- and non-nano-based products.
5 Conclusions
The group discussions revealed that there is still much to learn in the area of nanotoxicology. Slow progress is being made in characterizing and understanding the behavior of nanomaterials in biological systems; the development, diversity, and use of these materials, however, is rapidly surpassing our ability to assess their unintended impacts on humans and other biota using traditional in vivo mammalian systems. The complexity and variety of nanoparticles further confounds this paucity of information. As with toxicity assessment of other chemicals and drugs, in the near future there will still be reliance on some whole animal testing. With the advent of high-throughput screening using lower organisms such as zebrafish and C. elegans, however, it may be possible to identify highly bioactive nanomaterials in an early tier of testing. In the in terim, the development of mechanistically based in vitro tests will continue, and in the spirit of the vision expressed in the Tox-21c report, these tests will focus on pathways of toxicity. Similarly, discussions of the appropriate characterization of nanoparticles should continue along with the development of standards. Finally, and perhaps most importantly at this stage, a reliable, open, curated database should be developed that interfaces with databases that are currently being used in Europe and elsewhere to enable sharing of information using a unified format. It might be envisioned that, in the US, such a database would be administered at a government agency, in cooperation with existing European efforts. Since this workshop occurred, such a project has been initiated in the United States by several institutes within the NIH.
Acknowledgements
We thank the Doerenkamp-Zbinden Foundation for sponsoring this workshop as part of the t4 activities.
Footnotes
a report of t4 – the transatlantic think tank for toxicology.
N.B. The opinions in this manuscript represent those of the individuals and not the institutions, organizations or agencies for which they work.
References
- Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Daneshian M, Leist M, Hartung T. Center for Alternatives to Animal Testing – Europe (CAAT-EU): a trans atlantic bridge for the paradigm shift in toxicology. ALTEX. 2010;27:63–69. doi: 10.14573/altex.2010.1.63. [DOI] [PubMed] [Google Scholar]
- Hartung T. Food for thought … on alternative methods for nanoparticle safety testing. ALTEX. 2010;27:87–95. doi: 10.14573/altex.2010.2.87. [DOI] [PubMed] [Google Scholar]
- NNI. National Nanotechnology Initiative Strategic Plan, February, 2011. 2011 http://www.nano.gov/nnistrategicplan211.pdf.
- National Research Council. Toxicity testing in the 21st century – a vision and a strategy. Washington, DC: National Academies Press; 2007. [Google Scholar]