Abstract
Malaria, which is caused by Plasmodium parasite erythrocyte infection, is a highly inflammatory disease with characteristic periodic fevers caused by the synchronous rupture of infected erythrocytes to release daughter parasites. Despite the importance of inflammation in the pathology and mortality induced by malaria, the parasite-derived factors inducing the inflammatory response are still not well characterized. Uric acid is emerging as a central inflammatory molecule in malaria. Not only is uric acid found in the precipitated form in infected erythrocytes, but high concentrations of hypoxanthine, a precursor for uric acid, also accumulate in infected erythrocytes. Both are released upon infected erythrocyte rupture into the circulation where hypoxanthine would be converted into uric acid and precipitated uric acid would encounter immune cells. Uric acid is an important contributor to inflammatory cytokine secretion, dendritic cell and T cell responses induced by Plasmodium, suggesting uric acid as a novel molecular target for anti-inflammatory therapies in malaria.
Keywords: Malaria, Plasmodium, Uric acid, Uricase, Inflammation, Inflammatory, Cytokines, Innate immunity, Cerebral malaria, Plasmodium falciparum
Introduction
Malaria is a highly inflammatory disease commonly known to induce cyclical fever attacks. The disease is caused by infection with Plasmodium, a protozoan parasite that invades and replicates within erythrocytes, with each replicative cycle ending in erythrocyte rupture. As Plasmodium replication is synchronized within the host, the exit of the newly formed daughter parasites and other components from erythrocytes takes place almost simultaneously in all infected cells. Over 100 years ago, a link to host immune activation was drawn by Golgi who discovered that malaria fever occurs one to two hours after rupture of infected erythrocytes [1]. It was postulated that the characteristic paroxysms of malarial fevers are caused by ‘malaria toxins’ [2].
The host inflammatory response in malaria is a decisive factor in the outcome of the disease, with inflammation mediating parasite clearance but also contributing to severe malaria pathology. Excessive and persistent inflammation during Plasmodium falciparum infection contributes to the development of pathologies such as cerebral malaria and severe malarial anemia, which are major causes of death due to malaria [3–5].
Despite the crucial role that inflammation plays in malaria, the parasite-derived molecules that trigger it have not been conclusively identified. The host inflammatory response is believed to be triggered by pro-inflammatory molecules present in the parasite and/or the ruptured host infected erythrocyte. Some parasite pro-inflammatory molecules have been identified, including GPI-anchors [6], a parasite pigment called hemozoin [7, 8] and parasite DNA [9, 10]. Additional studies suggest that GPI-anchors do not contribute decisively to the innate immune stimulatory activity of Plasmodium [11], while Plasmodium DNA, bound to hemozoin [9] or to parasite histones [12] appears to be highly inflammatory. However, the relative contribution of these parasite molecules to the inflammatory response in malaria patients remains unclear [13].
Plasmodium-induced inflammation by uric acid. Studies in vitro
Uric acid is a physiological by-product of nucleic acid metabolism. The inflammatory characteristics of uric acid are well-known because of its pathological role in gout, where uric acid crystals formed in the synovial fluid cause a strong localized inflammatory response [14]. Uric acid has also been identified as a ‘danger signal’ to alert the immune response. Uric acid is released from dying cells in high quantities, which would promote crystallization within the local environment and inflammation [15].
Uric acid has recently emerged as an important mediator of malaria-induced inflammation. Plasmodium-infected erythrocytes accumulate hypoxanthine, a precursor for uric acid. Plasmodium cannot synthesize purines de novo and imports hypoxanthine from the extracellular environment as a purine source [16, 17], which accumulates during the late stages of infection [18••, 19•]. Imported hypoxanthine is not degraded into uric acid within the erythrocyte, since xanthine dehydrogenase activity, which converts hypoxanthine into uric acid, has not been detected in this cell type or in the Plasmodium parasite [20]. However, upon erythrocyte rupture and release into the extracellular medium, xanthine dehydrogenase, which is normally present in the blood [21], and whose expression is increased during Plasmodium infection [22••], will efficiently degrade it into uric acid. As proposed before for uric acid acting as a ‘danger signal’ [15], uric acid derived from degradation of Plasmodium hypoxanthine would promote crystallization within the local environment and inflammation [18••].
In addition to hypoxanthine, P. falciparum infected erythrocytes were found to accumulate uric acid precipitates in the cytosol of the intra-erythrocytic parasite, which are released to the extracellular environment together with daughter parasites upon erythrocyte rupture. Uric acid precipitates were also found in infected erythrocytes freshly isolated from patients with P. falciparum and P. vivax infections, in addition to different rodent malaria species, suggesting that uric acid precipitates are a conserved feature of Plasmodium spp. [23••].
It is important to remark that there is no increase in the total amount of uric acid present in infected erythrocytes [23••], suggesting that the activity of erythrocyte uric acid transporters [24] is not affected by infection; however high concentrations of hypoxanthine accumulate in infected erythrocytes [18••, 19•]. This is consistent with the absence of xanthine dehydrogenase activity in Plasmodium and in erythrocytes [20] and would imply that infection with Plasmodium induces precipitation of pre-existing uric acid within the erythrocyte, but not degradation of hypoxanthine into uric acid.
Upon rupture of infected erythrocytes, the release of uric acid precipitates in the circulation is expected to induce a strong inflammatory response. Fractionation of lysates of P. falciparum-infected erythrocytes showed that the activatory effect on human dendritic cells was concentrated in the pellet fraction and was partially sensitive to uricase and DNAse, confirming an essential role for both parasite DNA and uric acid in the inflammatory response to the parasite [23••].
P. falciparum-derived uric acid also contributes to the secretion of inflammatory cytokines, such as TNF, IL-1β and IL-6, by peripheral blood mononuclear cells in vitro [19•]. These inflammatory cytokines are increased in malaria patients and associated with severity of the disease [25, 26], suggesting again an inflammatory role for uric acid in malaria.
An additional role for uric acid in Plasmodium immune response was recently proposed, as precipitated uric acid induces the release of Flt3 ligand from mast cells, which in turn, induces the expansion of CD8α dendritic cells that drive the activation of CD8 T cells [22••]. Plasmodium accumulated hypoxanthine and uric acid precipitates are likely to play major roles in the human immune response to the parasite, not only as inflammatory activators [18••, 19•, 23••], but also as regulators of adaptive immune responses to the parasite [22••].
Uric acid in Plasmodium-induced inflammation. Studies in patients
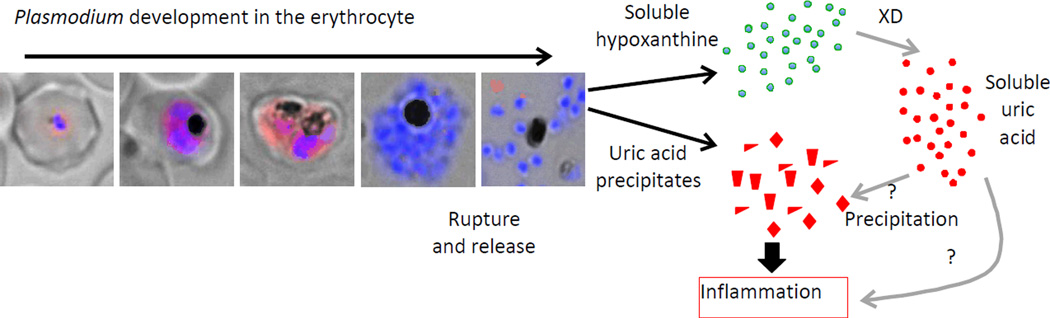
An important factor to consider in any uric acid-induced inflammatory response is the lack of uricase activity in humans and other higher primates. It is generally accepted that the loss of uricase was an adaptive change, since kidneys recover most of the filtered uric acid [27]. Uric acid is a strong antioxidant agent acting as both, radical scavenger and chelator of metal ions providing more than 50% of blood antioxidant ability [28]. The loss of uricase may be explained as a compensatory adaptation to the previous loss of the capacity to synthesize vitamin C, another antioxidant [29]. As a result of the lack of uricase activity, there is a moderated increase in serum uric acid level, reaching 2 – 4 mg/dL, which is found in primitive human cultures and apes with lack of uricase activity [30] and has increased to around 6 mg/dL in western cultures due to red meat enriched diets [31]. This level is very close to saturation and precipitation threshold in plasma > 7 mg/dL [32] and would create favorable conditions for precipitation of uric acid upon minor increases. This was proposed for dying cells releasing uric acid, which would act as an alarmin or ‘danger signal’ [15], and may possibly occur during a Plasmodium infection, where release of soluble hypoxanthine and uric acid, and subsequent formation of uric acid precipitates would result in increased uric acid concentrations in plasma [18••, 19•, 23••] (Figure 1).
Figure 1. Proposed model for uric acid-induced inflammation in malaria.
Plasmodium falciparum-infected erythrocytes at different stages of development show uric acid precipitates (red immunostaining). A more diffuse uric acid staining pattern is seen in early stage parasites, whereas a more distinct punctate pattern is seen in late stages and ruptured erythrocytes (blue=nuclei). Upon rupture, accumulated hypoxanthine is also released and converted into uric acid by Xanthine Dehydrogenase (XD). Precipitation of soluble uric acid may take place as a result of increased uric acid concentration and/or soluble uric acid may play a direct role in inflammatory response.
Elevated levels of plasma uric acid correlate with parasitemia and disease severity in P. falciparum infections [33–37]. In African children, plasma uric acid levels rose from 3.3 mg/dL to 4.60 mg/dL in uncomplicated malaria, reaching levels higher than 5.5 md/dL in severe malaria [36•, 37•]. These levels are also close to the precipitation threshold in plasma and could favor uric acid precipitation, as proposed above.
It is not clear whether the increase in uric acid in the circulation is due to impaired kidney function, a frequent complication in malaria [38], and/or to release of parasite accumulated hypoxanthine and uric acid precipitates. It is also possible that elevated uric acid is responsible for the kidney inflammation, as studies in mice have shown that hyperuricemia induced elevated levels of inflammatory cytokines and chemokines in the kidneys, in addition to T cell and macrophage infiltrations in the tubular interstitial space [39]. Regardless of the source, high levels of plasma uric acid in malaria patients correlate with high levels of inflammatory cytokines, such as IL-6, IL-10, sTNFRII, MCP-1, IL-8, TNFα and IP-10 in P. falciparum patients [36•], suggesting that uric acid may contribute to malaria severity by increasing the inflammatory host response.
A direct role for uric acid in malaria-induced inflammation is suggested by a clinical study in which administration of allopurinol, an inhibitor of xanthine dehydrogenase and therefore of the formation of uric acid, reduced inflammatory symptoms in malaria patients [40]. This study was performed to test the anti-Plasmodial activity of allopurinol, which is a purine analogue with activity against trypanosomatid parasites [41]. Despite an observed lack of anti-Plasmodial activity (also later documented in a mouse model [42]), P. falciparum-infected patients treated with allopurinol in addition to the anti-malarial drug quinine, had significantly faster decreases in fever and splenomegaly compared to patients treated with quinine alone [40]. Although the observed decrease in inflammatory symptoms might theoretically have resulted from a small decrease in the parasitemia observed in the allopurinol-treated group, rather than by the effects of allopurinol on uric acid levels, it is unlikely that a statistically non-significant decrease in parasite burden could induce a highly significant decrease (P<0.002) in inflammatory symptoms.
It is still under debate whether soluble uric acid levels in plasma have direct inflammatory effects on cells [43], whether the effect is mediated though uric acid precipitates that are formed in microenvironments as a result of the elevated levels of soluble uric acid or whether elevated uric acid is just a consequence of increased xanthine dehydrogenase activity, resulting in increased oxidative stress and inflammation [44]. In malaria, it appears that oxidative stress may not be a major cause for the strong inflammatory response, since treatment of patients with the anti-oxidant N-acetylcysteine did not significantly altered their cytokine profile [45].
Possible role of uric acid in cerebral malaria
Cerebral malaria is the most profound syndrome of severe malaria characterized by impaired consciousness, generalized convulsions, coma and neurological sequelae. Mature stage parasites express ligands, such as Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) on the surface of infected erythrocytes, that interact with host endothelial cell receptors such as intracellular adhesion molecule 1 (ICAM-1). This interaction leads to parasitized erythrocyte sequestration in the brain microcirculation, promoting the loss of endothelial cell junctions, endothelial apoptosis, and ultimately the disruption of the blood-brain barrier [5]. This disruption causes a massive diffusion of blood cells and serum into the brain tissue leading to coma and damage to the nervous system [46].
The interaction between P. falciparum infected erythrocytes and host endothelial cells is affected by inflammation, since cytokines such as TNFα cause increased expression of endothelial cell cytoadhesive surface proteins, such as ICAM-1, affecting the outcome of cerebral malaria [47].
Uric acid in humans may be either beneficial or detrimental to humans, depending on the cellular localization [48]. While uric acid has an extraordinary ability to scavenge radicals in the plasma and extracellular environment protecting cells from external oxidative stress, entry of uric acid into the cells has an opposite pro-oxidant effect [49]. Intracellular uric acid imported through specific organic anion transporters on the surface of endothelial cells decreases nitric oxide production by reducing arginine availability in endothelial cells leading to endothelial dysfunction [50, 51]. In humans, hyperuricemia appears to be a significant independent risk factor for endothelial dysfunction [52].
A role for uric acid in endothelial pathologies associated with P. falciparum infection was also recently suggested. In Malian children experiencing a malaria episode, increased plasma uric acid levels correlate not only with parasite density and disease severity, but also with markers of endothelial damage and dysfunction (soluble ICAM-1 and thrombomodulin) [36•]. Shedding of thrombomodulin that is normally bound to thrombin at the cell surface leads to an excess of active thrombin, which in turn induces platelet activation and aggregation [53]. Since platelet activation increases the binding of infected erythrocytes to endothelial cells, potentiating the cytotoxicity to the endothelium [54], it is possible that uric acid could contribute to endothelial damage through this mechanism.
Uric acid crystals, such as those found in gout, induce inflammation through the activation of the Nlrp3 inflammasome [55], but it is unclear whether Plasmodium-derived uric acid precipitates activate the same pathways in cerebral malaria. Several studies in mice suggest that the inflammasome plays a very limited role in the pathology of cerebral malaria. Mice deficient in either of the inflammasome components caspase-1, ASC adaptor, IL-1bβ IL-1b receptor and IL-18 succumbed to cerebral malaria [56, 57]. Yet, mice deficient in Nlrp3 presented a delayed onset of cerebral malaria. Additionally, a 600-fold increase in the expression of Nlrp3 mRNA of infected wt mice brain endothelial cells was found compared to uninfected controls, suggesting that Nlrp3 is involved in the pathogenesis of cerebral malaria in mice independent of the inflammasome, probably through the activation of non-classical pathways [56]. It is important to note that these results may be influenced by the differences in uric acid metabolism between mice and humans. If uric acid is a major trigger of the inflammatory response during malaria, the mouse model may not accurately reflect the human situation because, unlike humans, mice have active uricase in tissues that will rapidly degrade inflammatory uric acid precipitates [29].
Conclusions
Uric acid has emerged as a pathological factor in an increasing number of diseases, from classical gout to cardio-vascular diseases, including hypertension, renal failure and coronary disease [58]. In malaria, the activation of innate immunity is a major factor in the balance between parasite survival and host defense. The use of a ‘danger signal’ such as uric acid may be a consequence of co-evolution to exploit this warning system to trigger a high inflammatory response that could be crucial to Plasmodium survival, particularly in humans and primates that lack uricase activity. The strong inflammatory response to P. falciparum-derived uric acid suggests a novel molecular target for anti-inflammatory therapies in malaria.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Julio Gallego-Delgado, Maureen Ty, Jamie M. Orengo, Diana van de Hoef, and
Ana Rodriguez declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors. With regard to the authors’ research cited in this paper, all institutional and national guidelines for the care and use of laboratory animals were followed.
Contributor Information
Julio Gallego-Delgado, Email: Julio.GallegoDelgado@nyumc.org.
Maureen Ty, Email: MaureenMichelleTy@med.nyu.edu.
Jamie M. Orengo, Email: Jamie.Orengo@regeneron.com.
Diana van de Hoef, Email: Dvandehoef@nyas.org.
Ana Rodriguez, Email: ana.rodriguez@nyumc.org.
References
- 1.Golgi C. Sull Infezione malarica. Arch Sci Med (Torino) 1886;10:109–101. [Google Scholar]
- 2.Kwiatkowski D. Malarial toxins and the regulation of parasite density. Parasitol Today. 1995;11:206–212. doi: 10.1016/0169-4758(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 3.Clark IA, Cowden WB. The pathophysiology of falciparum malaria. Pharmacol Ther. 2003;99:221–260. doi: 10.1016/s0163-7258(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 4.Haldar K, Mohandas N. Malaria, erythrocytic infection, and anemia. Hematology Am Soc Hematol Educ Program. :200987–200993. doi: 10.1182/asheducation-2009.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Heyde HC, Nolan J, Combes V, et al. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 2006;22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coban C, Ishii KJ, Kawai T, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith JW, Sun T, McIntosh MT, Bucala R. Pure Hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol. 2009;183:5208–5220. doi: 10.4049/jimmunol.0713552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104:1919–1924. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, DeOliveira RB, Kalantari P, et al. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35:194–207. doi: 10.1016/j.immuni.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. J Immunol. 2010;184:4338–4348. doi: 10.4049/jimmunol.0903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowda NM, Wu X, Gowda DC. The nucleosome (histone-DNA complex) is the TLR9-specific immunostimulatory component of Plasmodium falciparum that activates DCs. PLoS One. 2011;6:e20398. doi: 10.1371/journal.pone.0020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdman LK, Finney CA, Liles WC, Kain KC. Inflammatory pathways in malaria infection: TLRs share the stage with other components of innate immunity. Mol Biochem Parasitol. 2008;162:105–111. doi: 10.1016/j.molbiopara.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther. 2010;12:206. doi: 10.1186/ar2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 16.Gero AM, O'Sullivan WJ. Purines and pyrimidines in malarial parasites. Blood Cells. 1990;16:467–484. discussion 485–498. [PubMed] [Google Scholar]
- 17.Asahi H, Kanazawa T, Kajihara Y, et al. Hypoxanthine: a low molecular weight factor essential for growth of erythrocytic Plasmodium falciparum in a serum-free medium. Parasitology. 1996;113(Pt 1):19–23. doi: 10.1017/s0031182000066233. [DOI] [PubMed] [Google Scholar]
- 18. Orengo JM, Evans JE, Bettiol E, et al. Plasmodium-induced inflammation by uric acid. PLoS Pathog. 2008;4:e1000013. doi: 10.1371/journal.ppat.1000013. This work shows for the first time that Plasmodium-derived uric acid induces an inflammatory response.
- 19. Orengo JM, Leliwa-Sytek A, Evans JE, et al. Uric acid is a mediator of the Plasmodium falciparum-induced inflammatory response. PLoS One. 2009;4:e5194. doi: 10.1371/journal.pone.0005194. This work shows inflammation by uric acid derived from the human parasite Plasmodium falciparum.
- 20.Reyes P, Rathod PK, Sanchez DJ, et al. Enzymes of purine and pyrimidine metabolism from the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 1982;5:275–290. doi: 10.1016/0166-6851(82)90035-4. [DOI] [PubMed] [Google Scholar]
- 21.Heath WR, Carbone FR. Immunology: dangerous liaisons. Nature. 2003;425:460–461. doi: 10.1038/425460a. [DOI] [PubMed] [Google Scholar]
- 22. Guermonprez P, Helft J, Claser C, et al. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med. 2013;19:730–738. doi: 10.1038/nm.3197. This work shows the role of uric acid in regulating the adaptive immune response to Plasmodium.
- 23. van de Hoef DL, Coppens I, Holowka T, et al. Plasmodium falciparum-derived uric acid precipitates induce maturation of dendritic cells. PLoS One. 2013;8:e55584. doi: 10.1371/journal.pone.0055584. This work shows the presence of inflammatory uric acid precipitates in human Plasmodium-infected erythrocytes.
- 24.Overgaard-Hansen K, Lassen UV. Active transport of uric acid through the human erythrocyte membrane. Nature. 1959;184:553–554. doi: 10.1038/184553b0. [DOI] [PubMed] [Google Scholar]
- 25.Grau GE, Piguet PF, Vassalli P, Lambert PH. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 26.Kwiatkowski D, Hill AV, Sambou I, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 27.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008;324:1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 28.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11:4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Lario B, Macarron-Vicente J. Uric acid and evolution. Rheumatology (Oxford) 2010;49:2010–2015. doi: 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ, Andrews P, Benner SA, Oliver W, Theodore E. Woodward award. The evolution of obesity: insights from the mid-Miocene. Trans Am Clin Climatol Assoc. 2010;121:295–305. discussion 305–308. [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson RJ, Titte S, Cade JR, et al. Uric acid, evolution and primitive cultures. Semin Nephrol. 2005;25:3–8. doi: 10.1016/j.semnephrol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Smyth CJ, Holers VM. Gout, hyperuricemia, and other crystal-associated arthropathies. New York: M. Dekker; 1999. p. 401. xvii. [Google Scholar]
- 33.Bertrand KE, Mathieu N, Inocent G, Honore FK. Antioxidant status of bilirubin and uric acid in patients diagnosed with Plasmodium falciparum malaria in Douala. Pak J Biol Sci. 2008;11:1646–1649. doi: 10.3923/pjbs.2008.1646.1649. [DOI] [PubMed] [Google Scholar]
- 34.Das BS, Patnaik JK, Mohanty S, et al. Plasma antioxidants and lipid peroxidation products in falciparum malaria. Am J Trop Med Hyg. 1993;49:720–725. doi: 10.4269/ajtmh.1993.49.720. [DOI] [PubMed] [Google Scholar]
- 35.Iwalokun BA, Bamiro SB, Ogunledun A. Levels and interactions of plasma xanthine oxidase, catalase and liver function parameters in Nigerian children with Plasmodium falciparum infection. Apmis. 2006;114:842–850. doi: 10.1111/j.1600-0463.2006.apm_457.x. [DOI] [PubMed] [Google Scholar]
- 36. Lopera-Mesa TM, Mita-Mendoza NK, van de, Hoef DL, et al. Plasma uric acid levels correlate with inflammation and disease severity in Malian children with Plasmodium falciparum malaria. PLoS One. 2012;7:e46424. doi: 10.1371/journal.pone.0046424. This work shows there is a correlation between inflammatory cytokines and levels of uric acid in malaria patients.
- 37. Mita-Mendoza NK, van de Hoef DL, Lopera-Mesa TM, et al. A potential role for plasma uric acid in the endothelial pathology of Plasmodium falciparum malaria. PLoS One. 2013;8:e54481. doi: 10.1371/journal.pone.0054481. This work shows a correlation between the levels of markers of endothelial cell stress and uric acid in malaria patients.
- 38.Mishra SK, Das BS. Malaria and acute kidney injury. Semin Nephrol. 2008;28:395–408. doi: 10.1016/j.semnephrol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, Fang L, Jiang L, et al. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS One. 2012;7:e39738. doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarma PS, Mandal AK, Khamis HJ. Allopurinol as an additive to quinine in the treatment of acute complicated falciparum malaria. Am J Trop Med Hyg. 1998;58:454–457. doi: 10.4269/ajtmh.1998.58.454. [DOI] [PubMed] [Google Scholar]
- 41.Marr JJ. Purine analogs as chemotherapeutic agents in leishmaniasis and American trypanosomiasis. J Lab Clin Med. 1991;118:111–119. [PubMed] [Google Scholar]
- 42.Gillman BM, Batchelder J, Flaherty P, Weidanz WP. Suppression of Plasmodium chabaudi parasitemia is independent of the action of reactive oxygen intermediates and/or nitric oxide. Infect Immun. 2004;72:6359–6366. doi: 10.1128/IAI.72.11.6359-6366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25:39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Jin M, Yang F, Yang I, et al. Uric acid, hyperuricemia and vascular diseases. Front Biosci (Landmark Ed) 2012;17:656–669. doi: 10.2741/3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watt G, Jongsakul K, Ruangvirayuth R. A pilot study of N-acetylcysteine as adjunctive therapy for severe malaria. QJM. 2002;95:285–290. doi: 10.1093/qjmed/95.5.285. [DOI] [PubMed] [Google Scholar]
- 46.Rasti N, Wahlgren M, Chen Q. Molecular aspects of malaria pathogenesis. FEMS Immunol Med Microbiol. 2004;41:9–26. doi: 10.1016/j.femsim.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 49.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz IF, Grupper A, Chernichovski T, et al. Hyperuricemia attenuates aortic nitric oxide generation, through inhibition of arginine transport, in rats. J Vasc Res. 2011;48:252–260. doi: 10.1159/000320356. [DOI] [PubMed] [Google Scholar]
- 51.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomiyama H, Higashi Y, Takase B, et al. Relationships among hyperuricemia, metabolic syndrome, and endothelial function. Am J Hypertens. 2011;24:770–774. doi: 10.1038/ajh.2011.55. [DOI] [PubMed] [Google Scholar]
- 53.Hanson SR, Harker LA. Interruption of acute platelet-dependent thrombosis by the synthetic antithrombin D-phenylalanyl-L-prolyl-L-arginyl chloromethyl ketone. Proc Natl Acad Sci U S A. 1988;85:3184–3188. doi: 10.1073/pnas.85.9.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Combes V, Coltel N, Faille D, et al. Cerebral malaria: role of microparticles and platelets in alterations of the blood-brain barrier. Int J Parasitol. 2006;36:541–546. doi: 10.1016/j.ijpara.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 56.Reimer T, Shaw MH, Franchi L, et al. Experimental cerebral malaria progresses independently of the Nlrp3 inflammasome. Eur J Immunol. 2010;40:764–769. doi: 10.1002/eji.200939996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kordes M, Matuschewski K, Hafalla JC. Caspase-1 activation of interleukin-1beta (IL-1beta) and IL-18 is dispensable for induction of experimental cerebral malaria. Infect Immun. 2011;79:3633–3641. doi: 10.1128/IAI.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanbay M, Segal M, Afsar B, et al. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99:759–766. doi: 10.1136/heartjnl-2012-302535. [DOI] [PubMed] [Google Scholar]