Table 1.
Biological data of activities of known compounds and synthesized molecules.
| Compound | Structure | rat FAAH IC50 (nM) |
human FAAH IC50 (nM) |
DRD3 EC50 (nM) |
DRD3% Efficacy* |
DRD2 EC50 (nM) |
DRD2% Efficacy** |
Ratio D2/D3 |
CB1 EC50 (nM) |
Ratio CB1/D3 |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 |
|
0.3 | 1.6 | 6.5 | 51.7 | >1000 | 41.2 | >154 | 0.9 | 0.1 |
| 8 |
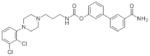
|
0.1 | 1.3 | 3.9 | 64.8 | 320.0 | 61.1 | 82 | 0.3 | 0.1 |
| 15 |
|
0.7 | 0.6 | 1.0 | 55.6 | 23.0 | 25.2 | 23 | 14.0 | 14 |
| 16 |
|
13.0 | 2.7 | 7.7 | 81.2 | 240.0 | 32.3 | 31 | 64.0 | 8 |
| 17 |
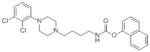
|
22.0 | 6.1 | 1.3 | 50.4 | 209.0 | A | 161 | 420.0 | 323 |
vs. 300 nM dopamine;
vs. 3 μM dopamine;
N.C., value not calculable, concentration – response curve show less than 25% effect at the highest concentration; A, antagonist
