Table 2.
Cross-coupling of Allylbenzene with Aryl bromides.
| entry | product | yield (%)[a] |
entry | product | yield (%)[a] |
|---|---|---|---|---|---|
| 1 | 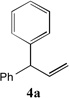 |
91[b] | 8 | 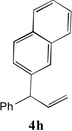 |
86[c] |
| 2 | 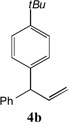 |
88[b] | 9 |
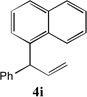
|
74[c] |
| 3 | 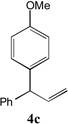 |
97[b] | 10 |
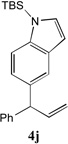
|
86[c,d] |
| 4 | 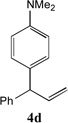 |
81[b] | 11 | 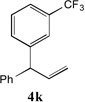 |
66[c,d] |
| 5 | 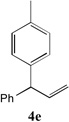 |
86[c] | 12 | 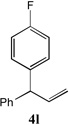 |
52[b] |
| 6 | 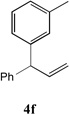 |
85[c,d] | 13 | 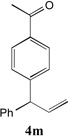 |
65[c,d] |
| 7 | 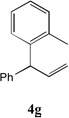 |
83[c,e] | 14 | 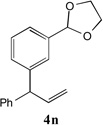 |
87[c,d] |
Less than 4 % of the gamma products were detected by NMR and no Heck product was observed under our conditions.
24 h.
36 h.
6 eq. of base used.
Concentration is 0.3M
