Table 3.
Cross-coupling of Allylbenzenes with Aryl bromides.
| entry | product | yield (%)[a] | entry | product | yield (%)[a] |
|---|---|---|---|---|---|
| 1 | 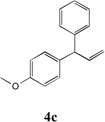 |
88 | 7 | 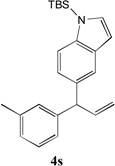 |
82 |
| 2 | 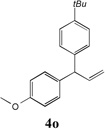 |
81 | 8 | 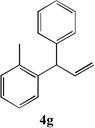 |
60[b] |
| 3 | 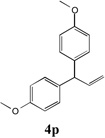 |
72 | 9 | 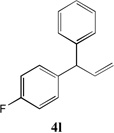 |
64 |
| 4 | 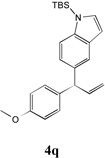 |
66[b] | 10 | 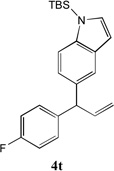 |
66 |
| 5 | 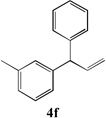 |
80[b] | 11 |
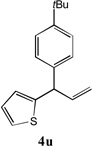
|
51[c] |
| 6 |
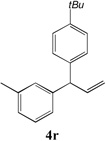
|
91 |
Reaction ran for 36 h.
6 eq. of base used.
5 eq. of thiophene allyl and 3 eq. of base used; obtained product along with 12 % of linear products.
