Summary
Background
The current surgical debate has led to a reduction in the extent of surgery performed and thereby to a reduced occurrence of surgical trauma and, over the recent years, reduced seroma formation. This reduction in surgical procedures calls the need for a drain into question.
Method
Using Google Scholar and the National Library of Medicine (PubMed), a literature review was performed on systematic reviews and meta-analyses regarding breast cancer surgery ± axillary dissection. Additionally, randomized trials for the time period after the last systematic review were included and evaluated according to the Jadad score.
Results
The search returned 5 systematic reviews, in which a total of 1,075 patients were included (537 cases and 538 controls). Since the last review, no prospective randomized trial meeting the inclusion criteria has been published. The current reviews conclude that insertion of a drain is associated with a longer hospital stay and reduced seroma formation. The data regarding wound infection and drain insertion is inconclusive. The omission of a drain is associated with early discharge, reduced postsurgical pain, and early mobilization, but also with an increase in outpatient seroma aspirations.
Conclusion
The omission of a drain is possible in early breast cancer surgery (wide local excision and sentinel node biopsy) with adequate surgical techniques and instruments.
KeyWords: Breast cancer, Surgery, Drain, Wound, Seroma, Sentinel, Breast conserving
Introduction
Breast cancer surgery (BCS) is one of the main treatment forms for early breast cancer. It combines the removal of the tumor (mastectomy, breast-conserving surgery, or wide local excision (WLE)) with axillary lymphnodectomy (ALNE) or sentinel node biopsy (SNB). Due to the increased knowledge of tumor genesis, earlier diagnosis, and improved systemic treatments, the extent of the surgical procedure has been reduced. Recently, WLE with SNB has been accepted as appropriate treatment for the majority of patients. Current guidelines [1] recommend resection of the tumor with a minimum of 1 mm tumor-free margin to be classified as R0. The surgical trauma is thereby reduced. Despite this reduction, surgeons still routinely place drains in the breast and axilla wounds. Common reasons for the insertion of a drain in early BCS are: (1) controlling a possible postsurgical hematoma, (2) drainage of the wound seroma, or (3) prevention of surgical-site infections (SSIs).
With the insertion of a drain these side effects should be positively influenced. Studies have shown that the omission of a drain is associated with a shorter hospital stay and less postsurgical pain, but also with an increase in seroma aspirations [2, 3, 4, 5, 6]. In the past, various risk factors like breast size, age, blood pressure, and number of tumor-infiltrated lymph nodes have been evaluated [2, 3]. Despite this, the medical benefit of a drain after BCS has not been established. In light of the American College of Surgeons Oncology Group Z0011 [7] study, which questions the need for ALNE, we ask if there is still a need for a wound drain in early BCS. Several reports [5, 6, 8] show that the omission of a drain is possible, and in some studies an influence on the number of wound infections [3, 9, 10, 11], increased pain [12, 13], and prolonged hospital stays [2, 13, 14, 15] are discussed as a result of drains after breast surgery. We looked for reviews regarding early BCS with and without drain, to identify evidence-based information on the omission of a wound drain. BCS includes mastectomy, breast-conserving surgery, biopsy (WLE), ALNE, and SNB dissection.
Materials and Methods
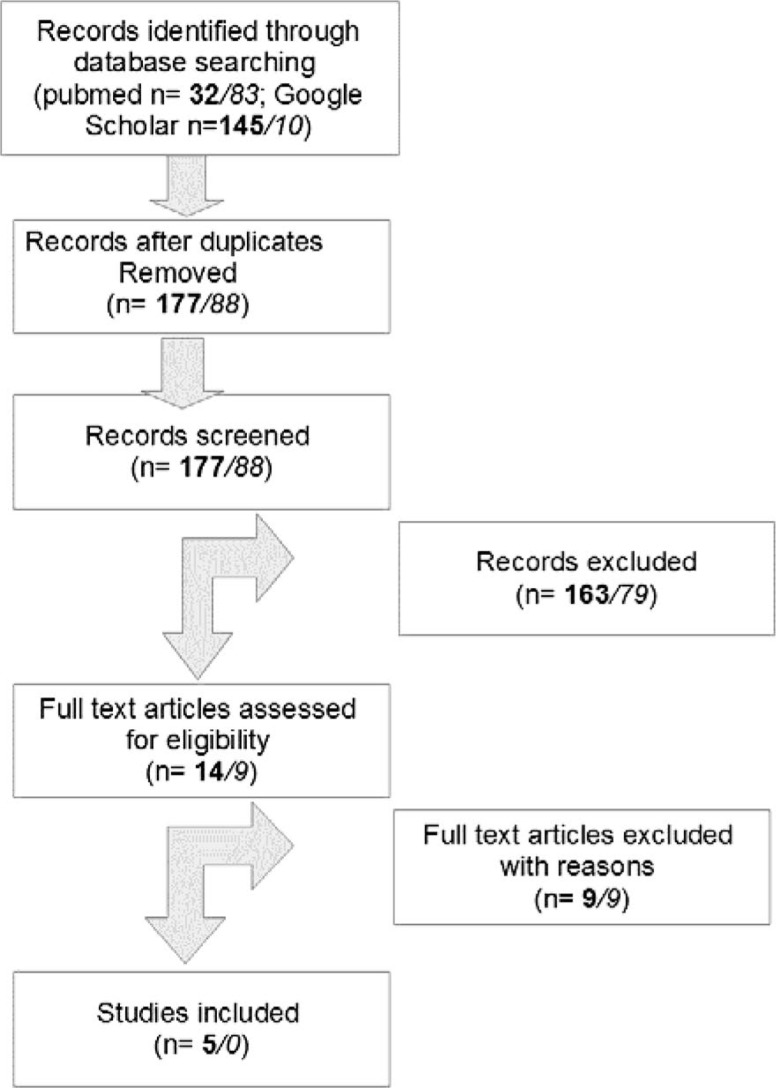
The literature in the National Library of Medicine (PubMed) and Google Scholar was searched for systematic reviews and meta-analyses regarding BCS ± axillary dissection and wound drainage. The search terms used were (Breast OR Mammary) AND (Cancer OR Carcinoma) AND (Drainage) AND (Surgery) AND English[lang] AND (Meta-Analysis[ptyp] OR systematic[sb]). The results were checked for relevance by title and summary (fig. 1). 5 publications were considered to be relevant to this topic by several of the authors (N.d.G., F.E., A.W.). Additionally, randomized trials from the time period after the last systematic review were included and evaluated according to a point system (Jadad score) by 2 of the authors (F.E., A.W.). The Jadad score reflects the quality of a study by adding points given for the randomization, dropout/withdrawal, and allocation concealment, as described in He et al. [4].
Fig. 1.
Literature selection (bold numbers for systematic reviews and meta-analyses; numbers in italics for randomized controlled trials).
Results
From the analysis, 9 articles were excluded for not answering the primary question. 3 articles investigated the impact of different instruments/fibrin glue on seroma production [16, 17, 18]. Other authors published on primary breast infections occurring without breast surgery or on breast wound dressing [19, 20]. The impact of physiotherapy or diet was researched, but again the intrasurgical drains were not considered [21, 22, 23, 24]. This left 5 systematic reviews relevant to the question, in which a total of 1,075 patients were included (537 cases and 538 controls). The same systematic approach was used for the time period after January 2012, looking for randomized controlled trails (fig. 1). Articles investigating different instruments/surgical techniques [25, 26, 27], postsurgical physiotherapy [28], or breast infections without relation to the insertion of drains [29, 30] were excluded. This left the 3 articles from Taylor et al. [5], Andeweg et al. [31], and Garbay et al. [32]. Taylor et al. [5] and Andeweg et al. [31] conducted retrospective studies and these articles were therefore also excluded. Garbay et al. [32] compared subgroups of 2 different independent prospective studies. This resulted in a low Jadad score and the article was therefore also excluded. Details regarding the included reviews are given in table 1.
Table 1.
Overview of the selected literature with details regarding the studies
| Review | Xue et al. [9] | Van Bemmel et al. [34] | He et al. [4] | Droeser et al. [2] | Kuroi et al. [11] |
|---|---|---|---|---|---|
| Number of trials | 8 RCTs | 136 trials | 6 RCTs | 6 RCTs | 51 RCTs, 7 prospective, 7 retrospective |
| Relevant trials | Brewer et al. [33], Bertin et al. [41] | Zavotsky et al. [12], Cameron et al. [36], Somers et al. [37], Soon et al. [38] | Zavotsky et al. [12], Jain et al. [13], Classe et al. [15], Cameron et al. [36], Somers et al. [37], Soon et al. [38] | Zavotsky et al. [12], Jain et al. [13], Cameron et al. [36] | Zavotsky et al. [12], Jain et al. [13], Purushotham et al. [14], Cameron et al. [36], Somers et al. [37], Soon et al. [38] |
| Number of patients, cases/controlsa | 33/82 | 205/195 | 297/287 | 102/71 | 453/409 |
| Title | ‘Risk factors for SSI after breast surgery: a systematic review and meta-analysis’ | ‘Prevention of seroma after axillary dissection in breast cancer’ | ‘Whether drainage should be used after surgery for breast cancer?’ | ‘Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery’ | ‘Evidence-based risk factors for seroma formation in breast surgery’ |
| Evaluation of reviewed articles | N/R | Oxford guidelinesb | adapted Jadad score | adapted Jadad score | Oxford guidelinesb |
| Wound seromas | seroma (OR: 1.65) | LoE 2/3 | OR: 0.36 (95% CI, 0.16-0.81; P = 0.01) | volume-controlled drainage less likely to develop clinically relevant seromas (RR 0.44; CI 0.24-0.80) | increased in the no-drain group |
| Infection | postoperative drain (OR: 2.84) | N/R | OR: 0.67 (95% CI, 0.34-1.32; P = 0.25) | no difference RR 1.23 (CI 0.70-2.16) | N/R |
| Hematoma | hematoma (OR: 2.45) | N/R | N/R | N/R | N/R |
| Pain | N/R | N/R | N/R | N/R | N/R |
| Hospital stay | N/R | N/R | OR: 1.52 (95% CI, 0.36-2.68; P = 0.01) | patients randomized to volume-controlled drainage stayed significantly longer than patients randomized to no/short term | N/R |
| Used instruments | N/R | LoE 3 for electrocautery, ultrasound scissors, electrothermal bipolar vessel system | none excluded | excluding ultrasound cutting, laser scalpel | electrocautery, laser scalpel, argon diathermy, ultrasonic scalpel, ultrasonic scissors |
| Conclusion | ‘There were also some factors that could be intervened to reduce the risk, such as … postoperative drains, drainage time and second drainage tube placed.’ | ‘Suction drainage thought to reduce dead space through negative pressure, significantly lowers seroma formation after surgery. A single flat type drain with multiple channels used for 48 hours produces the best results.’ | ‘The data suggests although no drainage compared to drainage after ALNE lead to shorten duration of hospital stay and no increase of the incidence of wound infection, seromas occurred more frequently and larger volume seromas required more aspirations.’ | ‘… clinically relevant seromas occur more frequently in patients treated with no/shortterm drainage after ALNE for breast cancer surgery, … However no/short-term drainage compared to volumecontrolled drainage … does not lead to increase in wound infection and is associated with a shorter hospital stay.’ | ‘On the other hand, the following factors did not have a significant influence on seroma formation: the duration of drainage; hormone receptor status; … type of drainage … In contrast, as might have been expected, SNB reduced seroma formation.’ |
Cases/controls: SSI/no SSI or drain/no drain.
Oxford Center for Evidence-Based Medicine (www.cebm.net).
ALNE = Axillary lymphnodectomy, CI = confidence interval, LoE = level of evidence, N/R = not reported, OR = odds ratio, RCT = randomized controlled trial, RR = relative risk, SNB = sentinel node biopsy.
Hematoma
Xue et al. [9] report on hematomas as a risk factor for SSIs. The original study investigated risk factors for SSI and did not have a no-drain control group [33]. Our search did not return any further articles comparing drain/no drain and looking at postsurgical hematoma.
Seroma
According to Droeser et al. [2], He et al. [4], Kuroi et al. [11], and Van Bemmel et al. [34], the insertion of a drain reduces the amount of seroma produced. 2 reviews [2, 4] each included 6 studies [10, 12, 13, 15, 35, 36, 37, 38, 39], with 3 studies reviewed in both reviews [12, 13, 36]. He et al. [4] also reported the number of seroma aspirations. The 3 studies reviewed [13, 15, 36] found a highly significant difference. For axillary dissection, Kuroi et al. [11] report on a correlation of seroma production with the use of electrocautery and ultrasonic scissors, but not with other ‘modern’ instruments (laser scalpels, argon diathermy, and ultrasonic scalpels). Van Bemmel et al. [34] and the small study by Manouras et al. [40] showing no seroma production when using an electrothermal bipolar vessel sealing system report similar findings. Van Bemmel et al. [34] recommend removal of the drain after 48 h. Kuroi et al. [11] report inconclusive data for drain removal on day 5 or with minimum volume. Xue et al. [9] report a significantly increased risk of SSI correlating with the drainage time in 3 studies, but none of the studies was matched against a no-drain control group.
Infection
Xue et al. [9] demonstrate a significant association between drains, the number of drains, the time of removal, and wound healing complications, using the Center of Disease Control (CDC) selection criteria. They review 2 case control studies [41, 42] including various types of breast biopsies (including benign diseases). The results of 2 other reviews [2, 4] are inconclusive. Droeser et al. [2] do not provide a definition of infection and review 6 articles [10, 12, 13, 35, 36, 39]. 3 studies did compare short-term versus volume-controlled removal of the drain [10, 37, 39]. He et al. [4] report on 5 trials [12, 13, 15, 37, 38], with 3 of them using a definition of infection. Both reviews report no correlation for the infection rate. In all 3 reviews, the heterogeneity was not significant. The studies from Jain et al. [13] and Zavotsky et al. [12] are used in both reviews [2, 4].
Hospital Stay
He et al. [4] and Droeser et al. [2] reported a prolonged hospital stay if a drain was inserted. He et al. [4] analyzed 3 studies [13, 15, 36] while Droeser et al. [2] examined 5 studies [10, 13, 35, 36, 39]. Testing for heterogeneity, both reviews were highly significant. It needs to be mentioned that the studies of Jain et al. [13] and Cameron et al. [36] were considered in both reviews.
The other reviews do not provide data on hospital stay.
Pain
Droeser et al. [2] point out that 2 studies [12, 13] report significantly lower postsurgical pain in the omission group. He et al. [4] include another study [14] with details regarding postsurgical pain. The authors do not report any difference between the study groups. The heterogeneity is not reported. The other reviews do not provide data on postsurgical pain.
Type of Surgery
In table 1, the type of surgery in the study groups is listed. All except 1 trial [9] gave information on axillary surgery, with only 1 trial [11] including data on SNB. 1 trial looked only at BCS with ALNE [34]. This combination was also the standard procedure. Data regarding oncoplastic surgery was not found with our search terms.
Discussion
Drains have been inserted after breast surgery for a long time [43]. As the extent of the surgical procedure decreases due to neoadjuvant chemotherapy or as a consequence of studies questioning the current standard [7, 44], surgical procedures also need to be adapted. Our study provides an overview of systematic reviews with various aspects of drains and their benefits. The studies reviewed looked into infection, seroma formation, pain, and hospital stay, providing broad insights into the current state of BCS and drains.
Hematoma
For a surgeon, it is reassuring to have a drain in situ in order to control the postsurgical blood loss. Only 1 review provided data on hematomas [9]. Alternative options to monitor the postsurgical bleeding are a clinical examination or a breast ultrasound. The numbers of hematomas that need revision are not reduced due to a drain or external dressings [45, 46, 47]. A current review by Kosin et al. [48] only reports that the use of drains in breast biopsies causes a significant benefit with regard to hematomas. These results are based on studies done over 19 years ago, with very small patient numbers [6, 49, 50]. On the other hand, Kosin et al. [48] report that ‘breast reduction studies did not demonstrate a need for prophylactic drainage’ [51, 52, 53]. Intrasurgical hemostasis is essential to avoid postsurgical blood loss. From our point of view, it is good clinical practice not to complete a surgical operation if the hemostasis level is unsatisfactory. Thus, complications are easily avoided, and the level of hemostasis should be monitored routinely with the use of audits.
Seroma
Droeser et al. [2] and He et al. [4] disagree in their results. While Droeser et al. [2] state that the omission of a drain might be feasible for breast-conserving therapy and ALNE, He et al. [4] take a more conservative view. They find no significant difference in seroma production for breast-conserving surgery and mastectomy and note a significant heterogeneity between the individual trials. This is given as an explanation for the discrepancy. Several risk factors were identified but not adequately addressed in the reviews (i.e., body mass index (BMI), surgical instruments, delayed shoulder movement, etc.).
Van Bemmel et al. [34] analyze the known surgical risk factors and conclude that further studies combining the known factors are needed. 1 factor the authors agree on is the extent of the surgery and its influence on seroma formation. This is also shown in other studies [4, 11] comparing radical mastectomy with mastectomy, breast-conserving surgery, and WLE. The same principle applies to SNB and ALNE. A randomized controlled trial [54] showed seroma reduction on secondary ALNE. The reason behind this could be a decrease in surgical trauma and therefore a reduced disturbance in the lymphatic drainage, resulting in less seroma formation. The impact of the surgical technique and the possible adverse impact of a drain are demonstrated in the study by Garbay et al. [32]. Here, the drain placed with the surgical axillary padding technique increased the seroma formation. This may indicate that the role of the surgical technique is underestimated.
In general, the literature supports the use of ultrasonic scissors for axillary dissection. Surgeons should reduce the use of electrocautery in order to reduce seroma formation [3]. New surgical devices, like the electrothermal bipolar vessel sealing system, show promising results but need to be evaluated with a ‘no-drain’ control group [40, 55, 56].
Early (< 48 h) removal of the drain is supported in the review of Srivastava et al. [3]. From the authors’ point of view, there is sufficient data showing that optimal surgery results in less seroma formation [3, 14].
Infection
The case-control studies reviewed by Xue et al. [9] have a lower level of evidence (LoE) than the prospective trials reviewed by Droeser et al. [2] and He et al. [4]. The studies also use different definitions for infection. Therefore, we conclude that the data on BCS including ALNE shows no clear benefit for the omission/insertion of a drain. The prevention of an SSI is a multilevel task. Current data indicates that other factors might be more influential than the insertion/omission of a drain.
Hospital Stay
A prolonged hospital stay was reported by Droeser et al. [2] and He et al. [4] for the drain subgroups. 3 out of 5 studies [10, 35, 39] did not contain a ‘no-drain’ group in Droeser et al. [2]. He et al. [4] reviewed 3 studies [13, 15, 36], but even after excluding the low-quality study of Cameron et al. [36] the heterogeneity is still significant. With significant heterogeneity, these results are more likely to be biased. Other trials showed that discharge with or without a drain in situ is possible [10, 14, 35, 57]. From the authors’ point of view, an inserted drain does not prolong the hospital stay.
Pain
The use of a visual analog scale (VAS) to analyze pain is a well-accepted method. The significant benefit in 2 [12, 13] out of 3 studies [12, 13, 15] seems plausible, as the drain can hinder the movement of the arm. On the other hand, large seroma formation within the first postsurgical days can also cause discomfort and is a risk factor for SSI. Postsurgical pain can be managed by sufficient pain medication.
Types of Surgery
Breast-conserving surgery with ALNE was the standard procedure in the reviews. Nowadays, the standard is WLE with SNB. The WLE-and-SNB group is a subgroup of 1 review [11]. Other authors compared the surgical sites of breast surgery (mastectomy vs. modified radical mastectomy [3, 11, 34] or modified radical mastectomy vs. WLE [3, 34]). Most studies used a combination of breast-conserving surgery and axillary surgery. Data on oncoplastic surgery was not included. Recent publications indicate that drains can be omitted in breast-conserving surgery [5, 6, 8, 48, 51, 52, 53].
The following limitations need to be considered. Even though we used a wide range of search terms, it cannot be excluded that a review or a current prospective trial is still in press or was not found. The reviews found had a large overlap in their primary literature and showed partial significance with regard to heterogeneity. The quality of the primary studies displayed a wide range. The recommendations varied depending on the inclusion criteria of the reviews. The numbers of patients included in the studies were generally low. No study included patients with neoadjuvant treatment. Neoadjuvant chemotherapy (NAC) plays an increasing role in modern treatment [58]. With one benefit of NAC being a reduction in the surgical field, 1 author found increased seroma production for 6 NAC patients [59]. Further trials with sufficient patient numbers need to investigate this.
The genetic background of the patients has only been taken into consideration in 1 of the reviews [9], and the intrinsic subtypes of the carcinomas and lymphangiosis have not been mentioned in any of the studies. As the pathophysiology of seroma formation is not yet understood, this is an area that has not been adequately researched. Breast-conserving surgery plus ALNE was the most common procedure. With ALNE being a risk factor for more seroma formation compared to SNB, the results could overestimate the benefit of a drain [60]. With the Z0011 study [7] questioning the need for axillary dissection, a prospective multicenter trial is needed with WLE plus SNB as study group. This would help to identify the patients at risk of seroma or to find the best combination of surgical techniques, instruments, and postoperative care.
Conclusions
Seroma formation is reduced by smaller surgical sites (i.e. WLE and SNB). With the use of modern surgical instruments (i.e. ultrasonic scissors or the electrothermal bipolar vessel sealing system), seroma production seems to be further reduced. This enables the omission of a drain in early BCS. If drains are placed, early removal is encouraged by the current literature, but randomized prospective multicenter trials are needed to find the optimal time. Also, in further studies, the role of drains in oncoplastic surgery needs to be evaluated.
Disclosure Statement
The authors declare that they have no financial or personal competing interests and that there is no external funding source.
References
- 1.Kreienberg R, Kopp I, Albert U-S, Bartsch HH, Beckmann MW, Berg D, et al. Interdisziplinäre S3-Leitlinie und Nachsorge des Leitlinie. Ger Cancer Soc. 2012;7:32–45. [Google Scholar]
- 2.Droeser RA, Frey DM, Oertli D, Kopelman D, Baas-Vrancken Peeters MJ, Giuliano AE, et al. Volume-controlled vs no/short-term drainage after axillary lymph node dissection in breast cancer surgery: a meta-analysis. Breast. 2009;18:109–114. doi: 10.1016/j.breast.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava V, Basu S, Shukla VK. Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer. 2012;15:373–380. doi: 10.4048/jbc.2012.15.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He X-D, Guo Z-H, Tian J-H, Yang K-H, Xie X-D. Whether drainage should be used after surgery for breast cancer? A systematic review of randomized controlled trials. Med Oncol. 2011;28(suppl 1):S22–S30. doi: 10.1007/s12032-010-9673-2. [DOI] [PubMed] [Google Scholar]
- 5.Taylor JC, Rai S, Hoar F, Brown H, Vishwanath L. Breast cancer surgery without suction drainage: the impact of adopting a ‘no drains’ policy on symptomatic seroma formation rates. Eur J Surg Oncol. 2013;39:334–338. doi: 10.1016/j.ejso.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Warren HW, Griffith CD, McLean L, Angerson WJ, Kaye B, McElroy M. Should breast biopsy cavities be drained? Ann R Coll Surg Engl. 1994;76:39–41. [PMC free article] [PubMed] [Google Scholar]
- 7.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebner FK, Friedl TWP, deGregorio A, Reich A, Janni W, Rempen A. Does non-placement of a drain in breast surgery increase the rate of complications and revisions? Geburtshilfe Frauenheilkd. 2013;73:1128–1134. doi: 10.1055/s-0033-1351071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue DQ, Qian C, Yang L, Wang XF. Risk factors for surgical site infections after breast surgery: a systematic review and meta-analysis. Eur J Surg Oncol. 2012;38:375–381. doi: 10.1016/j.ejso.2012.02.179. [DOI] [PubMed] [Google Scholar]
- 10.Baas-Vrancken Peeters MJ, Kluit AB, Merkus JWS, Breslau PJ. Short versus long-term postoperative drainage of the axilla after axillary lymph node dissection. A prospective randomized study. Breast Cancer Res Treat. 2005;93:271–275. doi: 10.1007/s10549-005-5348-7. [DOI] [PubMed] [Google Scholar]
- 11.Kuroi K, Shimozuma K, Taguchi T, Imai H, Yamashiro H, Ohsumi S, et al. Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol. 2006;36:197–206. doi: 10.1093/jjco/hyl019. [DOI] [PubMed] [Google Scholar]
- 12.Zavotsky J, Jones RC, Brennan MB, Giuliano AE. Evaluation of axillary lymphadenectomy without axillary drainage for patients undergoing breast-conserving therapy. Ann Surg Oncol. 1998;5:227–231. doi: 10.1007/BF02303777. [DOI] [PubMed] [Google Scholar]
- 13.Jain PK, Sowdi R, Anderson AD, MacFie J. Randomized clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. Br J Surg. 2004;91:54–60. doi: 10.1002/bjs.4435. [DOI] [PubMed] [Google Scholar]
- 14.Purushotham AD, McLatchie E, Young D, George WD, Stallard S, Doughty J, et al. Randomized clinical trial of no wound drains and early discharge in the treatment of women with breast cancer. Br J Surg. 2002;89:286–292. doi: 10.1046/j.0007-1323.2001.02031.x. [DOI] [PubMed] [Google Scholar]
- 15.Classe J-M, Berchery D, Campion L, Pioud R, Dravet F, Robard S. Randomized clinical trial comparing axillary padding with closed suction drainage for the axillary wound after lymphadenectomy for breast cancer. Br J Surg. 2006;93:820–824. doi: 10.1002/bjs.5433. [DOI] [PubMed] [Google Scholar]
- 16.Currie A, Chong K, Davies GL, Cummins RS. Ultrasonic dissection versus electrocautery in mastectomy for breast cancer – a meta-analysis. Eur J Surg Oncol. 2012;38:897–901. doi: 10.1016/j.ejso.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Sajid MS, Hutson K, Kalra L, Bonomi R. The role of fibrin glue instillation under skin flaps in the prevention of seroma formation and related morbidities following breast and axillary surgery for breast cancer: a meta-analysis. J Surg Oncol. 2012;106:783–795. doi: 10.1002/jso.23140. [DOI] [PubMed] [Google Scholar]
- 18.Carless PA, Henry DA. Systematic review and meta-analysis of the use of fibrin sealant to prevent seroma formation after breast cancer surgery. Br J Surg. 2006;93:810–819. doi: 10.1002/bjs.5432. [DOI] [PubMed] [Google Scholar]
- 19.Trop I, Dugas A, David J, El Khoury M, Boileau J-F, Larouche N, et al. Breast abscesses: evidence-based algorithms for diagnosis, management, and follow-up. Radiographics. 2011;31:1683–1699. doi: 10.1148/rg.316115521. [DOI] [PubMed] [Google Scholar]
- 20.Walter CJ, Dumville JC, Sharp CA, Page T. Systematic review and meta-analysis of wound dressings in the prevention of surgical-site infections in surgical wounds healing by primary intention. Br J Surg. 2012;99:1185–1194. doi: 10.1002/bjs.8812. [DOI] [PubMed] [Google Scholar]
- 21.McNeely ML, Peddle CJ, Yurick JL, Dayes IS, Mackey JR. Conservative and dietary interventions for cancer-related lymphedema: a systematic review and meta-analysis. Cancer. 2011;117:1136–1148. doi: 10.1002/cncr.25513. [DOI] [PubMed] [Google Scholar]
- 22.Devoogdt N, Van Kampen M, Geraerts I, Coremans T, Christiaens M-R. Different physical treatment modalities for lymphoedema developing after axillary lymph node dissection for breast cancer: a review. Eur J Obstet Gynecol Reprod Biol. 2010;149:3–9. doi: 10.1016/j.ejogrb.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Shamley DR, Barker K, Simonite V, Beardshaw A. Delayed versus immediate exercises following surgery for breast cancer: a systematic review. Breast Cancer Res Treat. 2005;90:263–271. doi: 10.1007/s10549-004-4727-9. [DOI] [PubMed] [Google Scholar]
- 24.Kligman L, Wong RKS, Johnston M, Laetsch NS. The treatment of lymphedema related to breast cancer: a systematic review and evidence summary. Support Care Cancer. 2004;12:421–431. doi: 10.1007/s00520-004-0627-0. [DOI] [PubMed] [Google Scholar]
- 25.Iovino F, Auriemma PP, Ferraraccio F, Antoniol G, Barbarisi A. Preventing seroma formation after axillary dissection for breast cancer: a randomized clinical trial. Am J Surg. 2012;203:708–714. doi: 10.1016/j.amjsurg.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 26.Sakkary MA. The value of mastectomy flap fixation in reducing fluid drainage and seroma formation in breast cancer patients. World J Surg Oncol. 2012;10:8. doi: 10.1186/1477-7819-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böhm D, Kubitza A, Lebrecht A, Schmidt M, Gerhold-Ay A, Battista M, et al. Prospective randomized comparison of conventional instruments and the Harmonic Focus(®) device in breast-conserving therapy for primary breast cancer. Eur J Surg Oncol. 2012;38:118–124. doi: 10.1016/j.ejso.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Marcos AL, El Gaaied ABA, Ayed FB, Hassen SB, Zervoudis S, Navrozoglou I, et al. Lymphedema of the arm after surgery for breast cancer: new physiotherapy. Clin Exp Obstet Gynecol. 2012;39:483–488. [PubMed] [Google Scholar]
- 29.Brahmbhatt RD, Huebner M, Scow JS, Harmsen WS, Boughey JC, Harris AM, et al. National practice patterns in preoperative and postoperative antibiotic prophylaxis in breast procedures requiring drains: survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2012;19:3205–3211. doi: 10.1245/s10434-012-2477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degnim AC, Throckmorton AD, Boostrom SY, Boughey JC, Holifield A, Baddour LM, et al. Surgical site infection after breast surgery: impact of 2010 CDC reporting guidelines. Ann Surg Oncol. 2012;19:4099–4103. doi: 10.1245/s10434-012-2448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andeweg CS, Schriek MJ, Heisterkamp J, Roukema JA. Seroma formation in two cohorts after axillary lymph node dissection in breast cancer surgery: does timing of drain removal matter? Breast J. 2011;17:359–364. doi: 10.1111/j.1524-4741.2011.01099.x. [DOI] [PubMed] [Google Scholar]
- 32.Garbay J-R, Thoury A, Moinon E, Cavalcanti A, Palma MD, Karsenti G, et al. Axillary padding without drainage after axillary lymphadenectomy – a prospective study of 299 patients with early breast cancer. Breast Care (Basel) 2012;7:231–235. doi: 10.1159/000341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brewer VH, Hahn KA, Rohrbach BW, Bell JL, Baddour LM. Risk factor analysis for breast cellulitis complicating breast conservation therapy. Clin Infect Dis. 2000;31:654–659. doi: 10.1086/314021. [DOI] [PubMed] [Google Scholar]
- 34.Van Bemmel AJM, van de Velde CJH, Schmitz RF, Liefers GJ. Prevention of seroma formation after axillary dissection in breast cancer: a systematic review. Eur J Surg Oncol. 2011;37:829–835. doi: 10.1016/j.ejso.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Dalberg K, Johansson H, Signomklao T, Rutqvist LE, Bergkvist L, Frisell J, et al. A randomised study of axillary drainage and pectoral fascia preservation after mastectomy for breast cancer. Eur J Surg Oncol. 2004;30:602–609. doi: 10.1016/j.ejso.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Cameron AE, Ebbs SR, Wylie F, Baum M. Suction drainage of the axilla: a prospective randomized trial. Br J Surg. 1988;75:1211. doi: 10.1002/bjs.1800751222. [DOI] [PubMed] [Google Scholar]
- 37.Somers RG, Jablon LK, Kaplan MJ, Sandler GL, Rosenblatt NK. The use of closed suction drainage after lumpectomy and axillary node dissection for breast cancer. A prospective randomized trial. Ann Surg. 1992;215:146–149. doi: 10.1097/00000658-199202000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soon PSH, Clark J, Magarey CJ. Seroma formation after axillary lymphadenectomy with and without the use of drains. Breast. 2005;14:103–107. doi: 10.1016/j.breast.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Kopelman D, Klemm O, Bahous H, Klein R, Krausz M, Hashmonai M. Postoperative suction drainage of the axilla: for how long? Prospective randomised trial. Eur J Surg. 1999;165:117–120. doi: 10.1080/110241599750007289. discussion 121–122. [DOI] [PubMed] [Google Scholar]
- 40.Manouras A, Markogiannakis H, Genetzakis M, Filippakis GM, Lagoudianakis EE, Kafiri G, et al. Modified radical mastectomy with axillary dissection using the electrothermal bipolar vessel sealing system. Arch Surg. 2008;143:575–580. doi: 10.1001/archsurg.143.6.575. discussion 581. [DOI] [PubMed] [Google Scholar]
- 41.Bertin ML, Crowe J, Gordon SM. Determinants of surgical site infection after breast surgery. Am J Infect Control. 1998;26:61–65. doi: 10.1016/s0196-6553(98)70062-8. [DOI] [PubMed] [Google Scholar]
- 42.Rey JE, Gardner SM, Cushing RD. Determinants of surgical site infection after breast biopsy. Am J Infect Control. 2005;33:126–129. doi: 10.1016/j.ajic.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Moss JP. Historical and current perspectives on surgical drainage. Surg Gynecol Obstet. 1981;152:517–527. [PubMed] [Google Scholar]
- 44.Glechner A, Wöckel A, Gartlehner G, Thaler K, Strobelberger M, Griebler U, Kreienberg R. Sentinel lymph node dissection only versus complete axillary lymph node dissection in early invasive breast cancer: a systematic review and meta-analysis. Eur J Cancer. 2013;49:812–825. doi: 10.1016/j.ejca.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 45.O'Hea BJ, Ho MN, Petrek JA. External compression dressing versus standard dressing after axillary lymphadenectomy. Am J Surg. 1999;177:450–453. doi: 10.1016/s0002-9610(99)00089-6. [DOI] [PubMed] [Google Scholar]
- 46.Petrek JA, Peters MM, Cirrincione C, Thaler HT. A prospective randomized trial of single versus multiple drains in the axilla after lymphadenectomy. Surg Gynecol Obstet. 1992;175:405–409. [PubMed] [Google Scholar]
- 47.Bonnema J, van Geel AN, Ligtenstein DA, Schmitz PI, Wiggers T. A prospective randomized trial of high versus low vacuum drainage after axillary dissection for breast cancer. Am J Surg. 1997;173:76–79. doi: 10.1016/S0002-9610(96)00416-3. [DOI] [PubMed] [Google Scholar]
- 48.Kosins AM, Scholz T, Cetinkaya M, Evans GRD. Evidence-based value of subcutaneous surgical wound drainage: the largest systematic review and meta-analysis. Plast Reconstr Surg. 2013;132:443–450. doi: 10.1097/PRS.0b013e3182958945. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler MH, Lakhany Z. Breast biopsy: a trial of wound drainage. Am J Surg. 1976;131:581–582. doi: 10.1016/0002-9610(76)90016-7. [DOI] [PubMed] [Google Scholar]
- 50.Law NW, Johnson CD, Lamont PM, Ellis H. Drainage or suture of the cavity after breast biopsy. Ann R Coll Surg Engl. 1990;72:11–13. [PMC free article] [PubMed] [Google Scholar]
- 51.Wrye SW, Banducci DR, Mackay D, Graham WP, Hall WW. Routine drainage is not required in reduction mammaplasty. Plast Reconstr Surg. 2003;111:113–117. doi: 10.1097/01.PRS.0000037867.10862.80. [DOI] [PubMed] [Google Scholar]
- 52.Collis N, McGuiness CM, Batchelor AG. Drainage in breast reduction surgery: a prospective randomised intra-patient trial. Br J Plast Surg. 2005;58:286–289. doi: 10.1016/j.bjps.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Corion LUM, Smeulders MJC, van Zuijlen PPM, van der Horst CMAM. Draining after breast reduction: a randomised controlled inter-patient study. J Plast Reconstr Aesthet Surg. 2009;62:865–868. doi: 10.1016/j.bjps.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Olson JA, McCall LM, Beitsch P, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol. 2008;26:3530–3535. doi: 10.1200/JCO.2007.15.5630. [DOI] [PubMed] [Google Scholar]
- 55.Nespoli L, Antolini L, Stucchi C, Nespoli A, Valsecchi MG, Gianotti L. Axillary lymphadenectomy for breast cancer. A randomized controlled trial comparing a bipolar vessel sealing system to the conventional technique. Breast. 2012;21:739–745. doi: 10.1016/j.breast.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Cortadellas T, Córdoba O, Espinosa-Bravo M, Mendoza-Santin C, Rodríguez-Fernández J, Esgueva A, et al. Electrothermal bipolar vessel sealing system in axillary dissection: a prospective randomized clinical study. Int J Surg. 2011;9:636–640. doi: 10.1016/j.ijsu.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Horgan K, Benson EA, Miller A, Robertson A. Early discharge with drain in situ following axillary lymphadenectomy for breast cancer. Breast. 2000;9:90–92. doi: 10.1054/brst.2000.0142. [DOI] [PubMed] [Google Scholar]
- 58.Huober J, von Minckwitz G. Neoadjuvant therapy – what have we achieved in the last 20 years? Breast Care (Basel) 2011;6:419–426. doi: 10.1159/000335347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodworth PA, McBoyle MF, Helmer SD, Beamer RL. Seroma formation after breast cancer surgery: incidence and predicting factors. Am Surg. 2000;66:444–450. discussion 450–451. [PubMed] [Google Scholar]
- 60.Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–4321. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]