Figure 7.
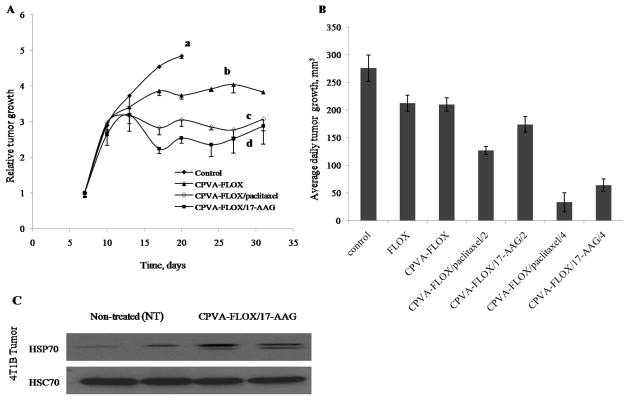
Tumor growth inhibition in mouse mammary carcinoma 4T1 model following the oral administration (gavage) of CPVA-FLOX nanogel or dual nanodrugs. (A) Relative tumor growth in BALB/c mice inoculated with 4T1 cells expressed as a ratio of tumor volume at any given time (V) to the tumor volume at the beginning of treatment (Vo). The treatment started on Day 7 after inoculation. (a) Control group: 2% sucrose, (b) CPVA-FLOX (dose: 16 mg/kg FLOX), (c) CPVA-FLOX/PCL (dose: 8mg/kg PCL, 16 mg/kg FLOX), and (d) CPVA-FLOX/17-AAG (dose: 16mg/kg 17-AAG, 16 mg/kg FLOX). (B) Average daily tumor growth calculated for the period of treatment between Days 10 and 17. The differences between CPVA-FLOX/PCL or CPVA-FLOX/17AAG-treated mice and control group were found significant (P<0.05). (C) HSP70 expression in 4T1 tumors after the oral treatment by CPVA-FLOX/17-AAG in the end of experiment on Day 32. Tumor cell lysates were analyzed by western blot. HSC70 protein served as internal control.
