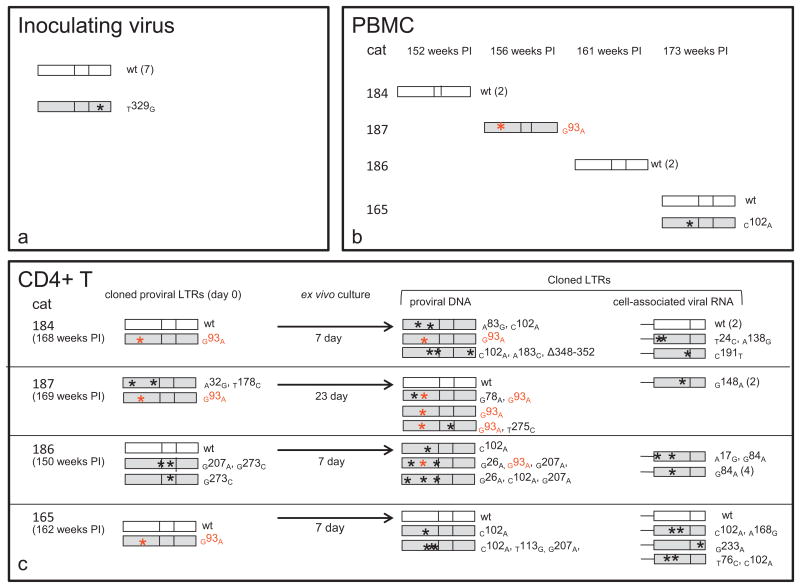
Fig. 5.
Viral promoter variants. LTR sequences were amplified, cloned, and then sequenced from the inoculating provirus and from PBMC or CD4 T cells isolated from the infected cats during the asymptomatic phase of infection. LTRs shown in white are identical to the inoculating virus sequence (wild type, wt) while shaded LTRs had at least one point mutation, approximate genomic position denoted by an asterisk. The number in parentheses next to the cloned sequences represents the number of copies sequenced of that exact same clone. The U3 point mutation in red (G93A) has been previously shown to be transcriptionally inactivating, whereas the C102A mutation had no effect on transcriptional activity of the viral promoter (Murphy et al., 2012). (a) Sequenced clones (8) of the inoculating provirus were identical except for a single SNP within the U5 region (T329G). (b) Mutations relative to the inoculating virus in the FIV proviral LTR at various time points PI in PBMC isolated from 4 FIV-infected cats. (c) Column-isolated, latently infected CD4T cells from 4 FIV-infected cats were cultured ex vivo for 7–23 days until viral reactivation was detected by RT-PCR. FIV gag amplicons were not detected by real-time RT-PCR assays in cDNA obtained from freshly isolated cells (day 0), but were detected on day 7 (cats 184, 186 and 165) or 23 (cat 187) of ex vivo culture. The FIV proviral LTR was cloned and sequenced before and after ex vivo reactivation. The U3/R region at the 3′ end of cell-associated vRNA was also cloned and sequenced after ex vivo reactivation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)