Figure 1.
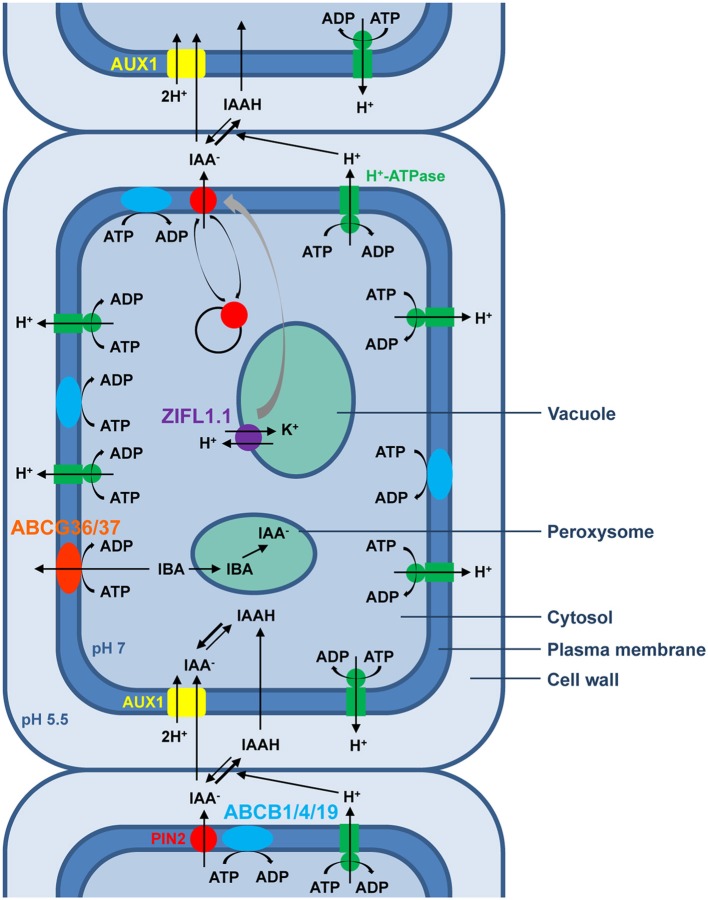
Schematic representation of polar auxin transport (PAT) in epidermal cells of the Arabidopsis root tip. According to the chemiosmotic hypothesis, the proton gradient generated primarily by plasma membrane H+-ATPases between the neutral cytoplasm and the acidic extracellular space drives the polarized auxin cell-to-cell movement. In the acidic apoplastic environment, a fraction of the weak acid IAA exists in its undissociated form, which can passively diffuse through the plasma membrane inside the cell. By contrast, the non-lipophilic and therefore less permeable proton-dissociated auxin fraction requires the amino acid permease-like AUX1, which catalyzes proton symport activity, to enter the cell. In the neutral cytosolic environment, IAA exists mainly in its membrane-impermeant anionic form that requires active transport to exit the cell. Hitherto, two distinct protein families whose members possess IAA-exporting activity have been associated with cellular polar auxin efflux. The best characterized auxin efflux carriers are members of the unique and plant-specific PIN protein family, believed to be secondary transporters energized by proton gradients. By contrast, some plant homologs of the human MDR/PGP transporters belonging to the ABCB subfamily, such as ABCB1, ABCB4, and ABCB19, have been implicated in ATP-energized auxin efflux. Although activity of ABCBs and the asymmetrical localization of AUX1 facilitates directionality of auxin transport, the bias, and rate of shootward auxin transport are mainly attributable to the highly regulated polar localization of the PIN2 transporter. Dynamic polar sorting of PIN2 at the plasma membrane is sustained by repeated steps of endocytic internalization and recycling back to the plasma membrane via exocytosis. In addition, potassium transport activity of the ZIFL1.1 tonoplastic carrier exerts a protective effect on PIN2 plasma-membrane stability. The hormonal activity of the auxin precursor IBA requires its conversion to IAA through β-oxydation in the peroxysome. Two members of the G-family of ABC transporters, ABCG36 and ABCG37, localize to the outward face of root epidermal cells and efflux IBA from root cells.