Figure 1.
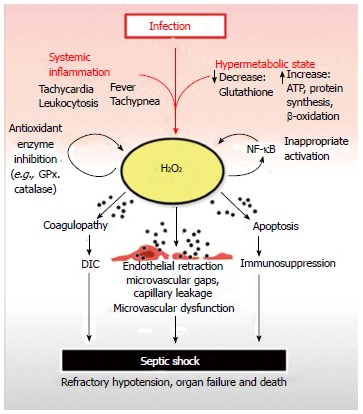
Septic shock begins with a systemic inflammatory reaction to an infection. A contemporaneous increase in metabolism is initiated, which can deplete reserves of critical nutrients such as glutathione. Glutathione is crucial for the neutralization of H2O2, a toxic, membrane-permeable oxidizing agent generated as a by-product of cellular metabolism. Depletion of cellular glutathione results in elevation of H2O2 which can diffuse out of organ parenchymal cells and into capillary endothelium before reaching the bloodstream. Once in the systemic circulation, excess H2O2 is distributed throughout the body resulting in systemic oxidative damage to plasma components, organs and blood vessels. The net result is H2O2 induced coagulopathy, immunocyte apoptosis and microvascular dysfunction leading to disseminated intravascular coagulation, immunosuppression, organ failure and septic shock respectively. H2O2 inhibits GPx and catalase, which are critical anti-oxidant enzymes required for H2O2 neutralization. This prevents restoration of normal plasma and tissue redox balance while exacerbating oxidative tissue damage. H2O2 can also activate nuclear factor-κB (NF-κB) contributing to the inappropriate activation of this master pro-inflammatory transcription factor observed in septic shock. The pathologic activation of NF-kB contributes to elevated tumor necrosis factor-alpha levels, another potent generator of intracellular H2O2. GPx: Glutathione peroxidase.
